Exhibit 10.1 COLLABORATION AND LICENSE AGREEMENT
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS,
HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE
COMMISSION PURSUANT TO RULE 24b-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS
AMENDED.
Exhibit 10.1
COLLABORATION AND LICENSE AGREEMENT
between
KIRIN BREWERY CO., LTD.,
(on the one hand)
and
MEDAREX, INC. and GENPHARM INTERNATIONAL, INC.
(on the other hand)
Effective as of September 4, 2002
TABLE OF CONTENTS
Page
ARTICLE I DEFINITIONS ............................................................... 2
ARTICLE II ANTIGEN AND ANTIBODY LISTS AND RELATED LISTING
PROCEDURES AND DISCLOSURE REQUIREMENTS .................................... 33
2.1 Antibody Sequence List and Antigen Lists Maintained by Each Party ...... 33
2.2 In-House Target Lists and Medarex Internal Project List ................ 33
2.3 Listing Procedures ..................................................... 33
2.4 Disclosure of Certain Antigen Lists .................................... 41
2.5 Confidentiality of Antigen and Antibody Disclosures .................... 41
2.6 Records Retention; [*] ................................................. 41
ARTICLE III ANTIBODY AND ANTIGEN AVAILABILITY ......................................... 42
3.1 Principles of Antibody and Antigen Availability ........................ 42
3.2 Medarex Antigen Inquiries .............................................. 43
3.3 Kirin Antigen Inquiries ................................................ 44
3.4 Medarex Antibody Inquiries ............................................. 48
3.5 Kirin Antibody Inquiries ............................................... 49
3.6 Notices, Inquiries and Other Requests Relating to Antibodies and
Antigens ............................................................... 51
3.7 Covenants Relating to Lists and Disclosures ............................ 52
3.8 Purchase of Rights ..................................................... 52
ARTICLE IV ANTIGEN AND ANTIBODY SELECTION FOR EXERCISE OF
LICENSE RIGHTS ............................................................ 52
4.1 Projects Involving Use of Medarex Mice and/or Medarex Technology ....... 52
4.2 Reservation Licenses ................................................... 53
4.3 Commercial Licenses .................................................... 54
4.4 [*] .................................................................... 62
ARTICLE V IN-HOUSE PROJECTS ......................................................... 64
5.1 In-House Target List ................................................... 64
5.2 [*] Target Selection ................................................... 67
5.3 [*] Target Selection ................................................... 68
5.4 Abandoned Target Selection ............................................. 69
5.5 Removing Targets from the [*] and the Target List ...................... 69
ARTICLE VI PARTNER PROJECTS .......................................................... 69
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-i-
TABLE OF CONTENTS
(continued)
Page
6.1 Partner Projects ...................................................... 69
6.2 Partner Projects Involving Special Relationships ...................... 70
6.3 [*] Advertisements or Other Promotions ................................ 70
6.4 Partner Opportunities ................................................. 71
6.5 Covenants ............................................................. 71
6.6 Offers of Collaboration Mice to Partners .............................. 73
ARTICLE VII CHANGE IN STATUS OF IN-HOUSE PROJECT OR PARTNER PROJECT .................. 74
7.1 Change in Rights Related to Target Designated for In-House Project .... 74
7.2 Change in Rights Related to Target Selected for Partner Project ....... 74
ARTICLE VIII GRANTS OF LICENSES ....................................................... 75
8.1 Present Grants of Licenses ............................................ 75
8.2 Medarex Licenses to Kirin ............................................. 75
8.3 Kirin Licenses to Medarex ............................................. 81
8.4 Negotiations Regarding Licenses Outside the Field ..................... 85
8.5 Sublicenses ........................................................... 85
8.6 Covenants Relating to Licenses and Sublicenses ........................ 91
8.7 Termination of Commercial License ..................................... 97
8.8 License Grants and Exercise Prior to the Effective Date ............... 97
8.9 Condition Precedent to Grant of a Commercial License .................. 99
8.10 Registration of Patent Licenses ....................................... 99
8.11 [*] and Rights Preserving Excuse ...................................... 99
8.12 No Other Rights ....................................................... 103
ARTICLE IX TRANSFER OF MATERIALS .................................................... 103
9.1 Transfer of Mice and Mice Materials ................................... 103
9.2 Information Disclosure ................................................ 105
9.3 Production Process Technology ......................................... 105
ARTICLE X LICENSE FEES, MILESTONES, ROYALTIES AND OTHER PAYMENTS ................... 105
10.1 Initial License Fees and Other Consideration .......................... 105
10.2 Reservation License Fees .............................................. 106
10.3 Commercial License Fees ............................................... 108
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-ii-
TABLE OF CONTENTS
(continued)
Page
10.4 Antigen Exclusivity Fees ......................................................... 109
10.5 [*] Target Milestone Payments .................................................... 111
10.6 In-House Project Royalties ....................................................... 117
10.7 Partner Royalties and Partner Payments ........................................... 118
10.8 Equity Investments by a Party in Connection with Partner Projects ................ 123
10.9 In-House Project Royalty, Partner Royalty and Partner Payment Terms .............. 125
10.10 Partner Payments and Royalty Payments Periods .................................... 126
10.11 Statements ....................................................................... 127
10.12 Records Retention; Audit ......................................................... 127
10.13 Mode of Payment and Withholding and Similar Taxes ................................ 128
10.14 Payments in Other Currencies ..................................................... 128
ARTICLE XI RESEARCH AND COLLABORATION RELATING TO JOINT TECHNOLOGY ............................... 129
11.1 Research and Research Reports .................................................... 129
11.2 Collaboration Meetings for Development of Medarex, Kirin and Joint Technologies .. 129
ARTICLE XII INTELLECTUAL PROPERTY RIGHTS .......................................................... 129
12.1 Intellectual Property Ownership .................................................. 129
12.2 Prosecution of Patents and Trademarks ............................................ 131
12.3 Enforcement of Patents ........................................................... 135
12.4 Infringement of Third Party Rights ............................................... 137
ARTICLE XIII CONFIDENTIALITY ....................................................................... 140
13.1 Disclosure and Use Restrictions .................................................. 140
13.2 Authorized Disclosure ............................................................ 140
13.3 Use of Name ...................................................................... 141
13.4 Press Releases and Filings with the SEC .......................................... 141
13.5 Equitable Relief ................................................................. 142
13.6 Third Party Agreements ........................................................... 142
ARTICLE XIV TERM AND TERMINATION .................................................................. 142
14.1 Term ............................................................................. 142
14.2 Termination of this Agreement for Material Breach ................................ 142
14.3 Consequences of Expiration or Termination ........................................ 143
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchan
ge Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-iii-
TABLE OF CONTENTS
(continued)
Page
14.4 Cure in Certain Instances ........................................................ 143
14.5 Rights in Bankruptcy ............................................................. 144
14.6 Accrued Rights; Surviving Obligations ............................................ 145
14.7 Notices Relating to Project Agreements for Kirin Partner Projects and
Kirin In-House Projects .......................................................... 146
ARTICLE XV INDEMNITY ............................................................................. 146
15.1 Indemnification of Medarex ....................................................... 146
15.2 Indemnification of Kirin ......................................................... 146
15.3 Indemnification Procedure ........................................................ 147
15.4 Insurance ........................................................................ 149
ARTICLE XVI REPRESENTATIONS AND WARRANTIES ........................................................ 149
16.1 Representations, Warranties and Covenants ........................................ 149
16.2 Additional Representations, Warranties and Covenants of Medarex .................. 150
16.3 Additional Representations, Warranties and Covenants of Kirin .................... 151
16.4 Knowledge; Officers .............................................................. 152
16.5 Limitation of Warranty ........................................................... 152
ARTICLE XVII DISPUTE RESOLUTION .................................................................... 152
17.1 General .......................................................................... 152
17.2 Interim Relief ................................................................... 153
17.3 Language ......................................................................... 153
17.4 Covenant Relating to "WHEREAS" Clauses ........................................... 153
ARTICLE XVIII MISCELLANEOUS ......................................................................... 153
18.1 Force Majeure .................................................................... 153
18.2 Governing Law .................................................................... 154
18.3 Notices .......................................................................... 154
18.4 Assignment ....................................................................... 156
18.5 Entire Agreement; Modifications .................................................. 157
18.6 Severability ..................................................................... 157
18.7 Waiver ........................................................................... 157
18.8 Independent Contractors .......................................................... 157
18.9 Language ......................................................................... 157
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchan
ge Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-iv-
TABLE OF CONTENTS
(continued)
Page
18.10 Descriptive Headings ............................................ 158
18.11 Counterparts .................................................... 158
18.12 No Third Party Rights ........................................... 158
18.13 Further Assurances .............................................. 158
18.14 Cumulative Remedies ............................................. 158
18.15 Attorneys' Fees ................................................. 158
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-v-
COLLABORATION AND LICENSE AGREEMENT
THIS COLLABORATION AND LICENSE AGREEMENT ("Agreement"), is made and entered
into as of September 4, 2002, by and between KIRIN BREWERY CO., LTD., having
principal offices at ▇▇-▇ ▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇-▇▇, ▇▇▇▇▇ ▇▇▇-▇▇▇▇, ▇▇▇▇▇
("Kirin") and MEDAREX, INC., having principal offices at ▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇
▇▇▇, ▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇-▇▇▇▇, ▇▇▇▇▇▇ ▇▇▇▇▇▇ of America, and a wholly
owned subsidiary, GENPHARM INTERNATIONAL, INC., with principal offices at ▇▇▇
▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇ ▇▇▇▇▇▇ of America
(collectively, "Medarex"). Kirin and Medarex each may be referred to herein
individually as a "Party," or collectively as the "Parties."
WHEREAS, Kirin, Medarex, and their respective Affiliates have developed,
and continue to refine and further develop, technologies in the area of
monoclonal antibody production utilizing mice carrying human immunoglobulin
loci;
WHEREAS, Medarex owns or otherwise has the right to license patent and
other intellectual property rights in and to a proprietary system for obtaining
high affinity monoclonal antibodies derived from mice carrying a portion of the
human immunoglobulin loci, a key component of which system is a transgenic mouse
referred to as the HuMAb Mouse(R) (as defined below);
WHEREAS, Kirin owns or otherwise has the right to license patent and other
intellectual property rights in and to a proprietary system for obtaining high
affinity monoclonal antibodies derived from mice carrying human immunoglobulin
loci, key components of which system are transchromosomic mice referred to as
the TC Mouse(TM) (as defined below) and the HAC Mouse(TM) (as defined below);
WHEREAS, Medarex and Kirin have collaborated to develop, and continue to
refine and further develop, a new proprietary system for obtaining high affinity
monoclonal antibodies derived from mice carrying a portion of the human
immunoglobulin loci, a key component of which system is a mouse referred to as
the KM-Mouse(TM) (as defined below);
WHEREAS, in the absence of the license grants contained in this Agreement,
Medarex believes that use or exploitation by Kirin of the TC Mouse(TM), HAC
Mouse(TM), HuMAb Mouse(R), or KM-Mouse(TM) and related information and
technology would infringe certain patent or other intellectual property rights
of Medarex, and Kirin believes that use or exploitation by Medarex of the TC
Mouse(TM), HAC Mouse(TM), or KM-Mouse(TM) and related information and technology
would infringe certain patent or other intellectual property rights of Kirin;
WHEREAS, while neither Kirin nor Medarex concedes that its use of such
technology would infringe any intellectual property rights of the other Party,
or concedes that there is any basis for any infringement claims by the other
Party, each Party nonetheless desires to avoid protracted and burdensome
litigation, and to secure licenses to any potential blocking patents of the
other Party that might prevent or hinder such Party from practicing and further
developing such technology, by entering this Agreement and granting each other
license rights to use and exploit the TC Mouse(TM), HAC Mouse(TM), HuMAb
Mouse(R), and KM-Mouse(TM) and related information and technology;
WHEREAS, subject to the terms and conditions of this Agreement, Medarex and
Kirin each desires the ability to grant to affiliates and/or third parties
licenses (and sublicenses) to exploit transgenic and/or transchromosomic mice
carrying at least a portion of the human immunoglobulin loci, specifically the
TC Mouse(TM), HAC Mouse(TM), HuMAb Mouse(R), KM-Mouse(TM) and related
information and technology, including rights to resulting products, so as to
allow affiliates and/or third parties to engage in research, development,
manufacture, and commercialization of such resulting products;
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
WHEREAS, affording each Party and its licensees access to and rights in
technology relating to the TC Mouse(TM), HAC Mouse(TM), HuMAb Mouse(R), and
KM-Mouse(TM) is likely to result in research and development synergies leading
to more rapid development and commercialization of marketable products;
WHEREAS, subject to the terms and conditions of this Agreement, Medarex and
Kirin each desires to grant to the other Party licenses to practice transgenic
and/or transchromosomic mouse technology, as the case may be, including that
technology related to the HuMAb Mouse(R), TC Mouse(TM), HAC Mouse(TM), and
KM-Mouse(TM) to generate antibodies derived from human immunoglobulin loci
carried by such mice in order to develop and commercialize antibody products
raised against and with affinity for antigens selected by Medarex, Kirin, or
their respective sublicensees;
WHEREAS, the Parties acknowledge and agree, in the spirit of equal
partnership, that they intend to provide each Party with access to the other
Party's Technology (as defined below) in a manner which encourages commercial
use by both Parties, consistent with the terms and conditions of this Agreement;
and
WHEREAS, Medarex and Kirin entered into that certain agreement entitled
"Agreement on Essential Terms for Collaboration," effective as of December 27,
1999, concerning the terms that form the basis of this Agreement, and the
Parties now desire to supersede such agreement with this Agreement.
NOW, THEREFORE, in accordance with the foregoing and for other valuable
consideration, the receipt and adequacy of which are hereby acknowledged, Kirin
and Medarex hereby agree as follows:
ARTICLE I
DEFINITIONS
The following capitalized terms used herein shall have the respective
meanings set forth below:
1.1 "1st Approval" shall mean, with respect to any Collaboration Product,
the first Marketing Approval obtained in any of the United States, a Major
European Country, or Japan.
1.2 "2nd Approval" shall mean, with respect to any Collaboration Product
as to which the 1st Approval has been obtained in any of the United States, a
Major European Country, or Japan, the immediately succeeding Marketing Approval
obtained with respect to such product in any of such territories in which the
1st Approval was not obtained. For the avoidance of doubt, in the event the 1st
Approval with respect to the Collaboration Product has been obtained in a Major
European Country for purposes of Section 1.1 hereof, in order for any succeeding
Marketing Approval with respect to such product to constitute the 2nd Approval
for purposes of this Section 1.2, such succeeding Marketing Approval must be
obtained in either the United States or Japan.
1.3 "3rd Approval" shall mean, with respect to any Collaboration Product
for which the 1st Approval and 2nd Approval have been obtained in any of the
United States, a Major European Country, or Japan, the immediately succeeding
Marketing Approval obtained with respect to such product in any of such
territories in which neither the 1st Approval nor the 2nd Approval was obtained.
For the avoidance of doubt, in the event that either the 1st Approval or 2nd
Approval with respect to the Collaboration Product has been obtained in a Major
European Country for purposes of Section 1.1 or Section 1.2 hereof, as
applicable, in order for any succeeding Marketing Approval with respect to such
product to constitute the 3rd Approval for purposes of this Section 1.3, such
succeeding Marketing Approval must be obtained
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-2-
in either the United States or Japan, depending on in which of such territories
such product has not obtained either the 1st Approval or the 2nd Approval.
1.4 "[*]" shall mean any Target [*] a Party from its In-House Target List
pursuant to Section 5.4 hereof.
1.5 "Acting Party" shall mean the Party other than the Responsible Party,
which acts to ▇▇▇▇▇ any Third Party infringement that relates to the KM Patent
Rights or KM Trademarks in accordance with Section 12.3.1(b) hereof in the event
that the Responsible Party elects not to act to ▇▇▇▇▇ such infringement.
1.6 "Additional Antibody Inquiry Response Period" shall have the meaning
set forth in Section 3.5.2(a) hereof.
1.7 "Additional Antigen Inquiry Response Period" shall have the meaning
set forth in Section 3.3.1(d)(i) hereof.
1.8 "Affiliate" shall mean with respect to any Person, another Person
that, directly or indirectly, through one or more intermediaries, controls, is
controlled by, or is under common control with such first Person. For purposes
of this definition only, "control" and, with correlative meanings, the terms
"controlled by" and "under common control with," shall mean (a) the possession,
directly or indirectly, of the power to direct the management or policies of a
Person, whether through the ownership of voting securities or by contract
relating to voting rights or corporate governance; or (b) the ownership,
directly or indirectly, of at least fifty percent (50%) of the voting securities
or other ownership interest of a Person; provided that, if local law requires a
minimum percentage of local ownership of voting securities or other ownership
interest, control will be established by direct or indirect actual or beneficial
ownership of the majority of the maximum ownership percentage that may, under
such local law, be owned by foreign interests; provided further that, except as
otherwise expressly provided in Sections 8.5.3(a) and 8.6.4 hereof, a Person
which is a Former Affiliate shall be deemed to be an Affiliate throughout the
Term of this Agreement with respect to any rights granted to or exercised by
such Person pursuant to a Former Affiliate Agreement. Notwithstanding the
foregoing, any agreement entered into by [*] prior to the Effective Date
(whether or not amended after the Effective Date but solely to the extent any
such amendment does not result in [*] shall be deemed not to be an agreement
between [*] and an "Affiliate" (or a "Former Affiliate" (as defined below)) for
purposes of this Agreement. With respect to [*], the Parties acknowledge and
agree that, as of the Effective Date, the facts and circumstances surrounding
[*] to satisfy the definition of an "Affiliate" or a "Former Affiliate" provided
in this Section 1.8; provided, however, that should [*] satisfy the definition
of an "Affiliate" provided in this Section 1.8 at any time after the Effective
Date, it shall be deemed an "Affiliate" for purposes of this Agreement (subject
to the preceding sentence).
1.9 "Agreement on Essential Terms for Collaboration" shall mean the
Agreement on Essential Terms for Collaboration entered into by and between
Medarex and Kirin effective as of December 27, 1999, as amended.
1.10 "Antibody" shall mean any human sequence monoclonal antibody, or any
fragment thereof or antibody fusion protein produced therefrom, with a unique
amino acid sequence that has a useful binding affinity for the Antigen against
which it was raised. For the purposes of this Agreement, each Antibody shall be
identified by reference to the Antibody Amino Acid Sequence, and Antibodies with
different Antibody Amino Acid Sequences (even if differing by only a single
amino acid) shall be deemed to be different Antibodies, irrespective of whether
they have affinity for the same Antigen.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-3-
1.11 "Antibody Amino Acid Sequence" shall mean, with respect to any
Antibody, the amino acid sequence [*].
1.12 "Antibody Availability Inquiry" shall mean a written inquiry provided
by Kirin to Medarex to ascertain the availability of an Antibody on behalf of
Kirin, any of its Affiliates, Partners or In-House Collaborators and which
describes the Antibody Amino Acid Sequence for the Antibody and the Antigen
against which the Antibody was raised, and for which the Antibody has affinity
(which Antigen description shall include the information that Kirin would be
required to provide to Medarex in accordance with this Agreement in the context
of an Antigen Availability Inquiry).
1.13 "Antibody Material" shall mean, (a) with respect to any Antibody, the
nucleic acids (including DNA, RNA, and complementary and reverse complementary
nucleic acids thereto, whether coding or noncoding and whether intact or a
fragment) in each case that code for such Antibody and do not code for multiple
Antibodies, or (b) a host cell (other than a host cell obtained directly from
Collaboration Mice or parts of such mice) into which the nucleic acids described
in (a) are introduced.
1.14 "[*]" shall mean, with respect to each Commercial License under which
a Party has the right pursuant to the terms and conditions of Article IV hereof
to exercise rights with respect to a particular Commercial Target, [*] as the
case may be, with respect to such Commercial License.
1.15 "Antibody Product" shall mean any composition or formulation
containing one or more Antibodies.
1.16 "Antibody Sequence List" shall mean a list identifying each (a)
Antibody that Medarex has selected, prior to the Effective Date, or selects on
or after the Effective Date, as the subject of a HuMAb Project (and with respect
to which Medarex desires to reserve rights for the exercise thereof by Medarex
or the grant of a license by Medarex to a Medarex Affiliate or a Third Party);
and (b) Selected Antibody; provided, however, that with respect to any Antibody
(including any Selected Antibody) with respect to which Medarex has granted a
license or other rights pursuant to an agreement entered into with a Third Party
prior to the Effective Date, the list shall identify only those Antibodies (or
Selected Antibodies, as the case may be) that have been or are disclosed to
Medarex in writing by such Third Party. For the avoidance of doubt, with respect
to Antibodies selected after the Effective Date, the Antibody Sequence List will
contain such items which are added in accordance with Section 2.3.2(b)(iii)
hereof.
1.17 "Antigen" shall mean any protein (including any glyco- or
lipo-protein or any other modified protein), peptide, carbohydrate, compound or
other composition, and any fragment or epitope of any of the foregoing, that
stimulates the production of Antibodies.
1.18 "Antigen Availability Inquiry" shall mean a written inquiry provided
by Kirin to Medarex to ascertain the availability of an Antigen on behalf of
Kirin, any of its Affiliates, Partners or In-House Collaborators, which
describes such Antigen by indicating all of the following information to the
extent that such information is [*] with respect to such Antigen at the time of
such inquiry: [*], other [*], the [*], equivalent [*] from other [*], and the
[*] and/or the [*] therefor; provided, however, that if there is no [*] or
equivalent [*] from other [*] with respect to such Antigen, Kirin shall describe
such Antigen by indicating, at a minimum, the [*] and/or [*] therefor.
1.19 "Antigen Evaluation Materials" shall mean, with respect to an
Antigen: (a) a written description of the Antigen, including [*]; (b) the [*]
and/or [*] for such Antigen; (c) data reasonably necessary for determining
whether such Antigen [*]; (d) [*] data in the possession of the Designating
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-4-
Party and/or its Affiliates relating to such Antigen; (e) information regarding
the [*] of such Antigen, the intellectual property rights Controlled by the
Designating Party and/or its Affiliates with respect to such Antigen, and any
[*] that would limit or otherwise affect the Parties' [*] any Collaboration
Products with respect thereto; (f) existing and available [*] for [*]; (g) a
list of [*] for Antibody Products against such Antigen; (h) [*] applicable to
Antibody Products against such Antigen; (i) [*] undertaken by or on behalf of
the Designating Party or its Affiliates (or its In-House Collaborator) with
respect to the [*] of [*] against such Antigen; (j) the [*] such Antigen is a
[*] for the development of Antibody Products; (k) [*] and the [*] regarding the
Antigen; and (l) other [*] in the Designating Party's or its Affiliates'
possession with respect to such Antigen.
1.20 "Antigen Exclusive Commercial License" shall mean a Medarex Antigen
Exclusive Commercial License or a Kirin Antigen Exclusive Commercial License, as
the case may be.
1.21 "Antigen Exclusivity Fee" shall mean the amount, if any, payable by a
Party to the other Party pursuant to Section 10.4.1, 10.4.2 or 10.4.3 hereof, as
applicable.
1.22 "Antigen Lists" shall mean any one or more of the Medarex Restricted
Antigen List, Shared Exclusive Antigen List, Medarex Non-Exclusive Antigen List,
and the Kirin Non-Exclusive Antigen List.
1.23 "Antigen Non-Exclusive Commercial License" shall mean a Medarex
Antigen Non-Exclusive Commercial License or a Kirin Antigen Non-Exclusive
Commercial License, as the case may be.
1.24 "Antigen Semi-Exclusive Commercial License" shall mean a Medarex
Antigen Semi-Exclusive Commercial License or a Kirin Antigen Semi-Exclusive
Commercial License, as the case may be.
1.25 "Asia" shall mean the dark shaded territory on the map attached as
Exhibit A.
1.26 "Bankruptcy Code" shall mean the Title 11 of the United States Code.
1.27 "Breeding License" shall mean the Medarex Breeding License or the
Kirin Breeding License, as the case may be.
1.28 "Calendar Quarter" shall mean each period of three consecutive
calendar months ending on March 31, June 30, September 30, and December 31.
1.29 "Calendar Year" shall mean each successive period of twelve months
commencing on January 1 and ending on December 31.
1.30 "Coding System" shall mean the system to be implemented by Medarex
pursuant to Section 2.3.1(c) hereof whereby Medarex assigns a unique code to
each Antigen and each Antibody, as the case may be, when placing the Antigen on
an Antigen List or the Antibody on the Antibody Sequence List, as applicable,
pursuant to Section 2.3.2 hereof.
1.31 "Collaboration Antibody" shall mean an Antibody that, during the
period beginning on December 27, 1999, and ending on the last day of the Term,
has been originally obtained, isolated, identified, or derived from one or more
of the Collaboration Mice, whether or not subsequently refined or produced
through the use of nucleic acids or cells obtained, isolated, identified, or
derived from one or more of the Collaboration Mice; provided, however, that no
Antibody originally obtained, isolated,
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-5-
identified, or derived from a Medarex Mouse, or through the use of nucleic acids
or cells obtained, isolated, identified, or derived from a Medarex Mouse, by or
on behalf of Medarex, a Medarex Affiliate, a Medarex Partner, an In-House
Collaborator or other Third Party to which Medarex has granted rights with
respect to Medarex Mice, shall be deemed to be a Collaboration Antibody.
1.32 "Collaboration Mouse" shall mean a Kirin Mouse, Medarex Mouse,
or KM-Mouse(TM), and "Collaboration Mice" shall mean more than one Collaboration
Mouse.
1.33 "Collaboration Product" shall mean any Antibody Product
containing one or more Collaboration Antibodies.
1.34 "Commercialization Rights" shall mean, with respect to any
Collaboration Antibody or Collaboration Product comprising such Antibody (or
Antibody Material related thereto), the rights to sell, lease, offer to sell or
lease, or otherwise transfer title to such Collaboration Antibody or
Collaboration Product.
1.35 "Commercial License" shall mean an Antigen Exclusive Commercial
License, an Antigen Semi-Exclusive Commercial License, or an Antigen
Non-Exclusive Commercial License, as the case may be.
1.36 "Commercial License Fee" shall mean the amount, if any, payable,
by a Party to the other Party pursuant to Section 10.3.1(a) or 10.3.1(b) hereof,
as applicable.
1.37 "Commercial Target" shall mean an Exclusive Commercial Target, a
Semi-Exclusive Commercial Target, or a Non-Exclusive Commercial Target, as the
case may be.
1.38 "Confidential Information" of a Party shall mean any and all
information and any tangible embodiments thereof in any medium provided by or on
behalf of such Party to the other Party, either in connection with the
discussions and negotiations pertaining to the Agreement on Essential Terms for
Collaboration or this Agreement or in the course of performing the Agreement on
Essential Terms for Collaboration or this Agreement, including, without
limitation, data, knowledge, practices, processes, ideas, research plans,
engineering drawings, research data, techniques, scientific, marketing and
business plans, and financial and personnel matters relating to the disclosing
Party or to its present or future products, sales, suppliers, customers,
employees, investors, or business. Notwithstanding any of the foregoing,
information shall not be deemed Confidential Information if it:
(a) was already known, discovered or developed by the receiving
Party or its Affiliates, other than under an obligation of confidentiality or
non-use owed by the receiving Party to the disclosing Party, prior to the time
of disclosure to the receiving Party;
(b) was generally available or known, or was otherwise part of
the public domain, prior to its disclosure to the receiving Party;
(c) became generally available or known, or otherwise became
part of the public domain, after its disclosure to the receiving Party through
no fault of the receiving Party;
(d) was disclosed to the receiving Party or its Affiliates,
other than under an obligation of confidentiality or non-use, by a Third Party
who had no obligation of non-disclosure or non-use to the disclosing Party; or
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-6-
(e) was independently discovered or developed by such receiving
Party or its Affiliates, as evidenced by their written records, without access
to the Confidential Information of the disclosing Party.
1.39 "Contracting Party" shall mean the Party (or the Affiliate of
which, as permitted under Section 8.5.3 hereof) that enters or is considering
entering into a Project Agreement with respect to a Partner Project pursuant to
which such Party (or any of its Affiliates) would receive or actually receives
securities from a Partner in connection with such Partner Project in accordance
with Section 10.8 hereof.
1.40 "Control" shall mean, with respect to any Information and
Inventions, Patent Rights or other intellectual property right, possession of
the right, whether directly or indirectly, and whether by ownership, license,
contract or otherwise (but not by grants of rights or licenses by Medarex and
Kirin to one another under this Agreement), to assign or grant a license,
sublicense or other right to or under such Information and Invention, Patent
Right or other intellectual property right as provided for herein.
1.41 "Core Third Party Patents" shall mean issued, unexpired Patent
Rights Controlled by a Third Party and [*] to [*] or [*] as of [*], that have
[*] in a [*] or [*] by a [*], which Patent Rights would [*] by a [*] (or [*])
[*] Mice, [*] Mice (to the extent mice having [*] existed on [*]) and/or [*] (to
the extent obtained by [*] having the [*] of such a [*] Mouse with a mouse
having the [*] of such a [*] MouseTM, which respective mice existed on [*]), but
[*] all (a) [*], (b) Patent Rights specifically directed to [*], and (c) Patent
Rights specifically directed to [*]. For purposes of this definition, "breeding"
shall include conventional methods of breeding and any and all methods for
combining genetic material from two or more different strains of mice into a
single mouse and shall not be limited to a single breeding generation.
1.42 "Cross License Agreement" shall mean that certain Cross License
Agreement entered into by and between Abgenix, Inc., Cell Genesys, Inc., Japan
Tobacco Inc. and Xenotech L.P., on the one hand, and GenPharm International,
Inc., on the other hand, effective as of March 26, 1997, as amended from time to
time, which amendment(s) is consistent with Section 16.2.2 hereof.
1.43 "Cure" shall have the meaning set forth in Section 8.11.4
hereof.
1.44 "Cure Period" shall mean (a) for purposes of Section 14.2
hereof, the [*] calendar day period, commencing [*], or (b) for purposes of
Section 8.11.4 hereof, the [*] business day period, commencing [*] pursuant to
Section 8.11.4 hereof, whichever may be applicable in the relevant context,
provided that in circumstances governed by Section 8.11.4 hereof, the term "Cure
Period" shall [*].
1.45 "[*]" shall have the meaning set forth in Section 1.1 of the
form of [*] Agreement agreed to in writing by the Parties on the date of
execution of this Agreement.
1.46 "Designated Exchange Rate" shall mean the Kirin Designated
Exchange Rate for purposes of calculating payments from Kirin to Medarex and
shall mean the Medarex Designated Exchange Rate for purposes of calculating
payments from Medarex to Kirin, in each case in accordance with the terms of
this Agreement.
1.47 "Designating Party" shall mean that Party which designates an
Antigen as a Target for inclusion on its In-House Target List.
1.48 "Designation Date" shall mean the date on which a Party
designates an Antigen as a Target on its In-House Target List pursuant to
Section 5.1.3(a), 5.1.3(b), or 5.1.3(d) hereof, as applicable.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-7-
1.49 "Diagnostic Application" shall mean an application of Antibody
Products to distinguish, identify and/or analyze a disease, genotype,
sensitivity or any other condition in a human, but excluding any In Vivo
Therapeutic Application and/or any Ex Vivo Therapeutic Application.
1.50 "Direct Sublicense Agreement" shall mean an agreement entered
into by Medarex and an Affiliate, Partner or In-House Collaborator of Kirin
pursuant to Section 8.5.1(d)(ii) of this Agreement, which agreement shall be in
the form agreed to in writing by the Parties on the date of execution of this
Agreement, except to the extent such form of agreement is modified in each case
to indicate the appropriate effective date and the counterparty's name, address,
and other relevant identifying information, and to the extent that Annex A
attached to a Direct Sublicense Agreement and made a part thereof is modified to
include appropriate information, in each case in accordance with the terms of
Section 8.5.1(d)(iii) hereof.
1.51 "[*]" shall have the meaning set forth in Section 2.6.3 hereof.
1.52 "[*]" shall have the meaning set forth in Section 2.6.3 hereof.
1.53 "Dispute" shall mean a dispute that arises between the Parties
in connection with or relating to this Agreement or the performance or breach of
any right or obligation hereunder.
1.54 "Dispute Notice" shall mean a written notice of a Dispute
provided by one Party to the other Party pursuant to Section 17.1.1 hereof.
1.55 "DNX Agreement" shall mean that certain license agreement
between DNX, Inc. and GenPharm International, Inc., effective as of January 1,
1991, as amended from time to time, which amendment(s) is consistent with
Section 16.2.2 hereof.
1.56 "Effective Date" shall mean the date first written in the
preamble to this Agreement.
1.57 "Embodiments of Intellectual Property" shall have the meaning
set forth in Section 14.5.2 hereof.
1.58 "[*]" shall mean the Third Party that acts as "[*]" under the
[*] Agreement entered into by the Parties with such Third Party or, where
applicable, a Third Party that acts as "[*]" under a [*] Agreement entered into
in accordance with Section 2.3.5(a) hereof.
1.59 "[*]" shall mean the agreement, substantially in the form agreed
to in writing by the Parties on the date of execution of this Agreement (which
form of agreement may be modified only by mutual written agreement of the
Parties), that is initially entered into by the Parties [*], or, where
applicable, any subsequent agreement, substantially in the form originally
agreed to in writing by the Parties on the date of execution of this Agreement,
that is entered into in accordance with Section 2.3.5(a) hereof and/or the terms
of the [*] Agreement.
1.60 "Evaluation License" shall mean the Medarex Evaluation License
or the Kirin Evaluation License, as the case may be.
1.61 "Excluded Claims" shall mean (a) Production Process Patents; and
(b) other claims in Patent Rights to the extent such claims are limited to (i) a
single Ig Composition, (ii) a single Antigen, (iii) antibodies, antibody
fragments, and/or antibody fusion proteins that have affinity for one or more
specified Antigen(s), (iv) hybridomas or cells expressing a single Ig
Composition, or (v) methods of Manufacture, development, combination
compositions, formulations, analytical methods, or use of Ig
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-8-
Compositions that have affinity for or encode a protein that has affinity for
one or more specified Antigen(s). Notwithstanding the foregoing, the claims in
the Patent Rights that were or are licensed to Medarex under the Cross License
Agreement shall not be deemed to be "Excluded Claims" to the extent that such
claims fall within clause (b)(iii) of the preceding sentence.
1.62 "Excluded Know-How" shall mean (a) Production Process Know-How;
and (b) Know-How to the extent such Know-How relates to (i) a single Ig
Composition, (ii) a single Antigen, (iii) antibodies, antibody fragments, and/or
antibody fusion proteins that have affinity for one or more specified
Antigen(s), (iv) hybridomas or cells expressing a single Ig Composition, or (v)
methods of Manufacture, development, combination compositions, formulations,
analytical methods, or use of Ig Compositions that have affinity for or encode a
protein that has affinity for one or more specified Antigen(s).
1.63 "Exclusive Commercial Target" shall mean a Kirin Exclusive
Commercial Target or a Medarex Exclusive Commercial Target, as the case may be.
1.64 "Expert" shall mean a disinterested individual who is free of
conflicts-of-interest, is not affiliated with either Party or any of either
Party's Affiliates, and has the relevant scientific, technical and/or regulatory
experience necessary to resolve a Dispute pursuant to Article XVII hereof or a
matter relating to Rights Preserving Excuse pursuant to Section 8.11 hereof, as
the case may be.
1.65 "Exploit" shall mean to do any one or more of the following:
research, develop, make, import, export, use, sell, offer for sale, and
otherwise exploit.
1.66 "Exploitation" shall mean doing any one or more of the
following: researching, developing, making, importation, exportation, use,
selling, offering for sale, and otherwise exploiting.
1.67 "Ex Vivo Therapeutic Application" shall mean the use of Antibody
Products that are applied in vitro to cells to alter the biological properties
of such cells, which cells are then provided, promoted and/or sold as
therapeutic and/or prophylactic agents for use in humans, including the use of
Antibody Products to separate cells from one another based on a specific
Antigen, but excluding any use for an In Vivo Therapeutic Application.
1.68 "[*] Target" shall mean any In-House Target, which is a
Commercial Target but is not a [*] Target.
1.69 "[*] Target Product" shall mean any Collaboration Product
containing a Collaboration Antibody(ies) raised against and with affinity for a
[*] Target.
1.70 "Field" shall mean any and all (a) In-Vivo Therapeutic
Applications and Ex-Vivo Therapeutic Applications; (b) Diagnostic Applications;
and (c) uses as a Reagent.
1.71 "First Commercial Sale" shall mean the first sale for use or
consumption of a Collaboration Product in a country or, if a Marketing Approval
is required in a country, the first sale for use or consumption of a
Collaboration Product in such country after Marketing Approval has been obtained
in such country.
1.72 "Former Affiliate" shall mean a Person which, with respect to a
Party, at one point in time satisfies the definition of an "Affiliate" contained
in Section 1.8 hereof, but which Person subsequently ceases to be an "Affiliate"
of such Party within the meaning of such definition.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-9-
1.73 "Former Affiliate Agreement" shall mean an agreement entered
into by a Party and a Person at a time when such Person satisfies the definition
of an "Affiliate" set forth in Section 1.8 hereof, but which Person subsequent
to entering into such agreement becomes a Former Affiliate of such Party.
1.74 "[*] Target" shall mean any In-House Target, which is a
Commercial Target, but with respect to which a Party [*] in connection with a
Collaboration Product(s) containing a Collaboration Antibody(ies) raised against
and with affinity for such Commercial Target.
1.75 "[*] Target Product" shall mean any Collaboration Product
containing a Collaboration Antibody(ies) raised against and with affinity for a
[*] Target.
1.76 "[*] Target Right" shall have the meaning set forth in Section
5.2.1 hereof.
1.77 "[*] Target Substitution" shall mean a Party's substitution for
a [*] Target of any other Target on such Party's [*] Target List, including,
without limitation, a Target that has been previously designated by such Party
as a [*] Target.
1.78 "[*] Target Substitution Fee" shall mean [*] in the case of
substitutions [*] and [*] in the case of substitutions [*].
1.79 "[*]" shall mean with respect to each Antigen that has been
selected by a Party as an Exclusive Commercial Target or a Semi-Exclusive
Commercial Target for the exercise of rights under an Antigen Exclusive
Commercial License or an Antigen Semi-Exclusive Commercial License, [*] with
respect to which the licensee Party (and/or its sublicensee) has a right to
exercise such License, which [*] is accomplished in accordance with Section
4.4.2 hereof, where applicable.
1.80 "[*]" shall mean, on a sublicense agreement-by-sublicense
agreement basis, the right of a Party to grant to a Third Party a sublicense
under an Evaluation License, [*].
1.81 "HAC MouseTM" shall mean any immunizable transchromosomic mouse
developed by or for Kirin and/or Kirin Affiliates, that contains one or more
human artificial chromosomes or fragments thereof that include centromere and
telomere sequences that provide for replication of such human artificial
chromosomes or fragments thereof in mice separate from replication of endogenous
mouse chromosomes, and which human artificial chromosome(s) or fragment(s)
comprises at least a portion of the unrearranged human immunoglobulin heavy
chain locus and at least a portion of an unrearranged human immunoglobulin light
chain locus; provided, however, that in no event shall a HuMAb Mouse(R),
KM-MouseTM or TC MouseTM be considered an HAC MouseTM for any purpose.
1.82 "Headquartered" shall mean with respect to a Person, the
location of such Person's principal offices from which the activities of the
Person are controlled and directed, or if such Person is under the control of an
Affiliate, the location of the principal offices from which the activities of
such Affiliate are controlled and directed.
1.83 "HuMAb Collaboration Project" shall mean a HuMAb Project as to
which (a) Medarex (and/or any Medarex Affiliate(s), as permitted under Section
8.5.3 hereof to the extent applicable) has entered into a written collaborative
research, development and/or commercialization agreement with one or more
Medarex Affiliate(s) or Third Party(ies) with respect to the Exploitation of the
Antibody Product(s) resulting from such project in exchange for Medarex (and/or
a Medarex Affiliate) receiving Substantial Commercialization Rights thereto;
provided, however, that Medarex's and/or a Medarex Affiliate's use of
subcontractors for research, development, or Manufacturing activities relating
to such
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-10-
project(s) shall not affect the characterization of a project as a "HuMAb
Collaboration Project" so long as Medarex and/or such Medarex Affiliate retains
Substantial Commercialization Rights with respect to Antibody Product(s)
resulting from such project; or (b) Medarex (and/or any Medarex Affiliate(s), as
permitted under Section 8.5.3 hereof to the extent applicable) has entered into
a material transfer or other technology evaluation agreement with one or more
Medarex Affiliate(s) or Third Party(ies) (other than an agreement that grants to
such Medarex Affiliate or Third Party(ies) a sublicense under a License granted
by Kirin to Medarex in this Agreement) for the purpose of determining whether
Medarex (and/or such Medarex Affiliate(s)) and such Medarex Affiliate(s) or
Third Party(ies) desire to conduct a project that meets the requirements set
forth in clause (a) of this Section.
1.84 "HuMAb License Project" shall mean a HuMAb Project as to which (a)
Medarex (and/or a Medarex Affiliate(s), as permitted under Section 8.5.3 hereof
to the extent applicable) has entered into a written agreement with one or more
Third Party(ies) with respect to such Third Party's(ies') Exploitation of the
Collaboration Product(s) resulting from such project whereby neither Medarex nor
any Medarex Affiliate retains any Substantial Commercialization Rights with
respect to such Collaboration Product(s); or (b) Medarex (and/or a Medarex
Affiliate(s), as permitted under Section 8.5.3 hereof to the extent applicable)
has entered into a material transfer or other technology evaluation agreement
with one or more Third Party(ies) for the purpose of determining whether such
Third Party(ies) desires to conduct a project that meets the requirements set
forth in clause (a) of this Section; provided, however, that a "HuMAb License
Project" shall not include any (i) other project conducted by Medarex (and/or a
Medarex Affiliate) with one or more Medarex Affiliates or Third Parties that
relates to one or more Antibodies, Antibody Materials and/or Antibody Products
initially developed and/or commercialized in connection with a Medarex Internal
Project or a HuMAb Collaboration Project even if such project provides to such
Medarex Affiliate or Third Party Substantial Commercialization Rights with
respect to the Antibody Product(s) resulting from such project and neither
Medarex nor any Medarex Affiliate retains Substantial Commercialization Rights
with respect to such Antibody Product(s); or (ii) Services Project.
1.85 "HuMAb Mouse(R)" shall mean any immunizable transgenic mouse
developed by or for Medarex and/or Medarex Affiliates that contains unrearranged
human immunoglobulin transgenes inserted into mouse chromosomes, but excluding
any such mouse that contains one or more human chromosomes or fragments thereof
that include human immunoglobulin loci and centromere and telomere sequences
that provide for replication of such human chromosomes or fragments thereof
separate from the replication of the endogenous mouse chromosomes; provided,
however that in no event shall a Kirin Mouse or KM-MouseTM be considered a HuMAb
Mouse(R) for any purpose.
1.86 "HuMAb Project" shall mean a research, development and/or
commercialization project that involves the use of any Medarex Mice, and/or an
exercise or grant of rights under any Medarex Technology (and, in Medarex's
discretion, the additional use of any Kirin Mice and/or KM-Mice, and/or an
exercise or grant of rights under any Kirin Technology and/or KM Patent Rights,
in each case only under the Medarex Evaluation License or a sublicense under the
Medarex Evaluation License in connection with the same project), to Exploit
and/or have Exploited Antibodies originally obtained, isolated, identified or
derived from such mice (and/or Antibody Materials related thereto and/or
Antibody Products containing such Antibodies) and which project is conducted by
Medarex, a Medarex Affiliate, and/or any Third Party with which Medarex has
entered into a written agreement with respect to such project; provided,
however, that no project that involves the Exploitation of Antibodies (and/or
Antibody Materials related thereto and/or Antibody Products containing such
Antibodies) raised against and with affinity for an Antigen with respect to
which Medarex, in connection with such project, has exercised rights under a
Reservation License or a Commercial License (and/or for which Medarex, in
connection with such project, has granted to a Third Party a sublicense under a
Reservation License or a Commercial
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-11-
License) shall be deemed a "HuMAb Project". For the avoidance of doubt, the
exercise by Medarex of rights under the Medarex Evaluation License in connection
with a project, or the grant by Medarex to an Affiliate or Medarex Affiliate
and/or Third Party of a sublicense under the Medarex Evaluation License in
connection with a project, shall not affect the status of the project as a
"HuMAb Project"; provided, however, that upon the exercise by Medarex of rights
or the grant by Medarex to an Affiliate or Third Party of a sublicense under a
Medarex Reservation License or a Commercial License (other than in connection
with a Services Project) granted to Medarex under this Agreement in connection
with a project, such project shall convert to a Medarex In-House Project or a
Medarex Partner Project, as the case may be.
1.87 "Ig Composition" shall mean an antibody, antibody fragment, or
antibody fusion protein characterized by a specific amino acid sequence spanning
heavy chain CDR1 through CDR3, or a nucleic acid encoding such antibody,
antibody fragment, or antibody fusion protein, wherein different molecules
having an identical amino acid sequence (or in the case of nucleic acid
molecules, those that code for the identical amino acid sequence) spanning heavy
chain CDR1 through CDR3 shall be considered to be the same single Ig
Composition. Ig Compositions having different amino acid sequences (or in the
case of nucleic acid molecules, those that code for different amino acid
sequence) spanning heavy chain CDR1 through CDR3 shall be considered to be
different Ig Compositions.
1.88 "Improvement" shall mean any modification to a Collaboration
Mouse, related mouse technology, or any discovery, device, or process relating
to such Collaboration Mouse or related mouse technology, whether or not patented
or patentable, including any enhancement in the efficiency, production or
operation of a Collaboration Mouse or related mouse technology, any discovery or
development of any new or expanded uses of a Collaboration Mouse or related
mouse technology, or any discovery or development that improves the stability,
safety, or efficacy of a Collaboration Mouse or related mouse technology, but in
any event excluding modifications, enhancements, discoveries, and developments
that are covered by or constitute Excluded Claims and/or Excluded Know-How.
1.89 "Indemnification Claim Notice" shall mean a written notice
provided by the Indemnified Party to the Indemnifying Party with respect to any
Losses or discovery of fact(s) upon which the Indemnified Party intends to base
a request for indemnification under Section 15.1 or Section 15.2.1 hereof and
which contains a description of the claim and the nature and estimated amount of
such Loss (to the extent that the nature and amount of such Loss is known at
such time).
1.90 "Indemnified Party" shall mean the Party that requests
indemnification under Section 15.1 or Section 15.2.1 hereof.
1.91 "Indemnifying Party" shall mean the Party asked to indemnify the
other Party under Section 15.1 or Section 15.2.1 hereof.
1.92 "Information and Inventions" shall mean technical, scientific and
other know-how and information, trade secrets, knowledge, technology, means,
methods, processes, practices, formulas, instructions, skills, techniques,
procedures, experiences, ideas, technical assistance, designs, drawings,
assembly procedures, computer programs, apparatuses, specifications, data,
results and other material, including pre-clinical and clinical trial results,
test procedures and purification and isolation techniques (whether or not
confidential, proprietary, patented, or patentable), in written, electronic or
any other form now known or hereafter developed, and all improvements thereto,
and other discoveries, developments, inventions and other intellectual property
(whether or not confidential, proprietary, patented or patentable).
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-12-
1.93 "In-House Collaborator" shall mean, with respect to any Project
Agreement that governs at least one In-House Project, in the case of Medarex, a
Third Party or a Medarex Affiliate with which Medarex (and/or a Medarex
Affiliate(s)) has entered or enters into such a Project Agreement, and in the
case of Kirin, a Third Party or a Kirin Affiliate with which Kirin (and/or a
Kirin Affiliate(s)) has entered or enters into such a Project Agreement, in each
case, whether prior to, on or after the Effective Date.
1.94 "In-House Project" shall mean a Medarex In-House Project or a
Kirin In-House Project, as the case may be.
1.95 "In-House Project Status Change" shall mean a change that a Party
makes in accordance with Section 7.1 hereof in the nature of such Party's
(and/or its Affiliate's) rights relating to an Antibody(ies) raised against and
with affinity for a particular Antigen (and Antibody Materials and Antibody
Products relating thereto) in connection with an In-House Project with the
result that neither such Party nor any of its Affiliates retains Substantial
Commercialization Rights relating to the applicable Antibody Product(s).
1.96 "In-House Project Status Change Date" shall mean the date on which
an In-House Project Status Change is implemented by a Party.
1.97 "In-House Target" shall mean an Antigen with respect to which
Medarex or Kirin, subject to the terms and conditions of Article IV hereof,
exercises its rights under a License in connection with an In-House Project.
1.98 "In-House Target List" shall mean, with respect to each Party, the
list that such Party maintains of its In-House Targets in accordance with
Section 2.2.1 hereof.
1.99 "Initial Fee" shall mean the Twelve Million Dollars (US
$12,000,000) initial fee paid by Kirin to Medarex in accordance with the
Agreement on Essential Terms for Collaboration.
1.100 "Invalidity Claim" shall have the meaning set forth in Section
12.4.2(d) hereof.
1.101 "Investigational New Drug Application" or "IND" shall mean an
investigational new drug application filed with the United States Food and Drug
Administration (and any successor agency thereto) for authorization to commence
human clinical trials, or such application's equivalent in countries or
regulatory jurisdictions other than the United States.
1.102 "In Vivo Therapeutic Application" shall mean the in vivo use of
Antibody Products for human therapeutic and/or prophylactic purposes where such
Antibody Products are delivered (by injection or otherwise) into the living
human body.
1.103 "Issuer" shall mean a Partner with which a Contracting Party
(and/or its Affiliate, as permitted under Section 8.5.3 hereof) intends to enter
or actually enters into a Project Agreement governing a Partner Project pursuant
to which such Contracting Party (and/or its Affiliate) may receive from such
Partner securities in connection with such Partner Project as contemplated by
Section 10.8 hereof.
1.104 "Japanese Partner" shall mean a Kirin Partner Headquartered in
Japan, which makes payments to Kirin in Japanese Yen.
1.105 "Kirin" shall mean Kirin Brewery Co., Ltd.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-13-
1.106 "Kirin Affiliate" shall mean an Affiliate of Kirin.
1.107 "Kirin Animal" shall have the meaning set forth in Section 8.2.5
(c) hereof.
1.108 "Kirin Antigen Exclusive Commercial License" shall mean each
license granted to Kirin by Medarex in Section 8.2.4(a) hereof.
1.109 "Kirin Antigen Non-Exclusive Commercial License" shall mean each
license granted to Kirin by Medarex in Section 8.2.4(b) hereof.
1.110 "Kirin Antigen Semi-Exclusive Commercial License" shall mean each
license granted to Kirin by Medarex in Section 8.2.4(c) hereof.
1.111 "Kirin Breeding License" shall mean the license granted to Kirin
by Medarex in Section 8.2.1 hereof.
1.112 "Kirin Commercial License" shall mean a Kirin Antigen Exclusive
Commercial License, a Kirin Antigen Semi-Exclusive Commercial License, or a
Kirin Antigen Non-Exclusive Commercial License, as the case may be.
1.113 "Kirin Commercial Target" shall mean a Kirin Exclusive Commercial
Target, a Kirin Semi-Exclusive Commercial Target, or a Kirin Non-Exclusive
Commercial Target, as the case may be.
1.114 "Kirin Designated Exchange Rate" shall mean the Telegram Transfer
Sell or TTS exchange rate published by the Bank of Tokyo-Mitsubishi Ltd., or, if
not so available, as otherwise mutually agreed by the Parties in writing.
1.115 "Kirin Evaluation License" shall mean the license granted to Kirin
by Medarex in Section 8.2.2 hereof.
1.116 "Kirin Exclusive Commercial Target" shall mean an Antigen with
respect to which Kirin exercises its rights under a Kirin Antigen Exclusive
Commercial License pursuant to and in accordance with Section 4.3.2,
4.3.3(d)(i), 4.3.3(e)(i) or 4.3.4 hereof; provided, however, that any such
Antigen shall cease to be a Kirin Exclusive Commercial Target upon the
expiration or termination of the Kirin Antigen Exclusive Commercial License
granted with respect to such Antigen.
1.117 "Kirin Improvement" shall mean any Improvement Controlled by
Kirin, but excluding any Improvements for which the Patent Rights or other
intellectual property rights are in-licensed by Kirin from a Third Party after
the Effective Date.
1.118 "Kirin In-House Project" shall mean a research, development
and/or commercialization project that involves the use of Medarex Mice, Kirin
Mice, and/or KM-Mice, and/or a grant of rights (including, without limitation, a
license or sublicense) under the Medarex Technology, Kirin Technology, and/or KM
Patent Rights, to Exploit and/or have Exploited Antibodies originally obtained,
isolated, identified or derived from such mice (and/or Antibody Materials
related thereto and/or Antibody Products containing such Antibodies) and as to
which (a) substantially all of the research or development effort will come from
Kirin's (and/or its Affiliate's) internal research or development assets and
Kirin (and/or a Kirin Affiliate) retains Substantial Commercialization Rights
with respect to the Antibody Product(s) resulting from such project; (b) Kirin
(and/or a Kirin Affiliate(s), as permitted under Section 8.5.3 hereof) has
entered into a written collaborative research, development and/or
commercialization agreement with one or more Kirin Affiliate(s) or Third
Party(ies) with respect to the Exploitation of the Antibody
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-14-
Product(s) resulting from such project in exchange for Kirin (and/or a Kirin
Affiliate) receiving Substantial Commercialization Rights thereto; or (c) Kirin
(and/or a Kirin Affiliate(s), as permitted under Section 8.5.3 hereof) has
entered into a material transfer or other technology evaluation agreement with
one or more Kirin Affiliate(s) or Third Party(ies) (other than an agreement that
grants to such Kirin Affiliate(s) or Third Party(ies) a sublicense under a
License granted by Medarex to Kirin pursuant to this Agreement) for the purpose
of determining whether Kirin (and/or a Kirin Affiliate(s)) and such Kirin
Affiliate(s) or Third Party(ies) desire to conduct a project that meets the
requirements set forth in clause (b) of this Section; provided, however, that
Kirin's (and/or a Kirin Affiliate(s)'s) use of subcontractors for research,
development, or Manufacturing activities relating to such project shall not
affect the characterization of a project as a "Kirin In-House Project" so long
as Kirin (and/or a Kirin Affiliate) retains Substantial Commercialization Rights
with respect to the Antibody Product(s) resulting from such project.
1.119 "Kirin In-License Agreements" shall mean the license agreements,
if any, listed on Exhibit D.
1.120 "Kirin Know-How" shall mean all Information and Inventions in the
Control of Kirin, during the period of time commencing on December 27, 1999, and
ending on the last day of the Term, and which Information and Inventions are
necessary for the use of the Kirin Mice, any Antibody derived from Kirin Mice,
or any Antibody Product containing such Antibody, and/or the performance of
activities covered by the Kirin Patent Rights and are not generally known, but
only to the extent conceived, reduced to practice or otherwise developed by one
or more employees or agents of Kirin and/or Kirin Affiliates, including, without
limitation: (a) biological, chemical, pharmacological, toxicological,
pharmaceutical, physical and analytical, clinical, safety and quality control
data and information necessary to reproduce, raise or use the Kirin Mice or
Antibodies, cells or nucleic acids originally obtained, isolated, identified, or
derived from one or more of the Kirin Mice; and (b) assays and methodologies
necessary for the use of the Kirin Mice; provided, however, that "Kirin
Know-How" excludes any Excluded Know-How, KM Know-How and any Information and
Inventions to the extent claimed by the Kirin Patent Rights.
1.121 "Kirin Materials" shall mean the Kirin Mice and/or KM-Mice
provided or to be provided by or on behalf of Kirin to Medarex or any Medarex
Affiliate pursuant to Section 9.1.1(a) hereof.
1.122 "Kirin Mouse" shall mean any (a) TC Mouse(TM); (b) HAC Mouse(TM);
(c) mouse generated by crossbreeding any TC MouseTM with any HAC MouseTM; and
(d) Improvement of a TC Mouse(TM), HAC Mouse(TM), or any mouse described in
clause (c) of this definition; provided, however, that in no event shall any
mouse classified as a Medarex Mouse or KM-Mouse(TM), or an Improvement of a
Medarex Mouse or KM-Mouse(TM) be deemed to be a "Kirin Mouse". "Kirin Mice"
shall mean more than one Kirin Mouse.
1.123 "Kirin Non-Exclusive Antigen List" shall mean a list identifying
each Antigen (other than an Antigen that is a ROFR Target) with respect to which
Kirin (a) from time to time during the Term exercises its rights under a
Reservation License and/or an Antigen Non-Exclusive Commercial License in
accordance with the terms and conditions of Article IV hereof; or (b) prior to
the Effective Date, in reliance on the Agreement on Essential Terms for
Collaboration, has entered into an agreement that provides for the grant to an
Affiliate or Third Party of a license or other rights (including, without
limitation, an option or a right of first refusal) in and to the Medarex Mice,
Kirin Mice and/or KM-Mice to raise Antibodies against such Antigen on an Antigen
non-exclusive basis.
1.124 "Kirin Non-Exclusive Commercial Target" shall mean an Antigen
with respect to which Kirin exercises its rights under a Kirin Antigen
Non-Exclusive Commercial License pursuant to and in
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-15-
accordance with Section 4.3.2, 4.3.3(d)(ii) or 4.3.4 hereof; provided, however,
that any such Antigen shall cease to be a Kirin Non-Exclusive Commercial Target
upon the expiration or termination of the Kirin Antigen Non-Exclusive Commercial
License granted with respect to such Antigen.
1.125 "Kirin Partner" shall mean, with respect to a Kirin Partner
Project, the Third Party with which Kirin (or a Kirin Affiliate, as permitted
under Section 8.5.3 hereof) has entered or enters into a Project Agreement
governing such Kirin Partner Project.
1.126 "Kirin Partner Payment" shall mean consideration of any type
received by Kirin (and/or any Kirin Affiliate(s)) from a Kirin Partner in
connection with a Kirin Partner Project, including, without limitation, up front
payments, license fees and milestones, but excluding amounts received in the
form of (a) royalty payments on sales of products; (b) amounts received, at or
below fair market value, for services provided by Kirin (or a Kirin Affiliate),
such as producing Antibodies and Manufacturing Antibody Products or conducting
research; (c) amounts received, at or below fair market value, for equity in
Kirin (or a Kirin Affiliate); (d) equity received from a Partner in exchange for
monetary consideration at or above fair market value; or (e) amounts received in
the form of a loan to Kirin (or a Kirin Affiliate) or repayment of a loan from
Kirin (or a Kirin Affiliate).
1.127 "Kirin Partner Project" shall mean a research, development
and/or commercialization project that involves the use of Kirin Mice, KM-Mice
and/or Medarex Mice, and/or a grant of rights (including, without limitation, a
license or a sublicense) under the Kirin Technology, Medarex Technology and/or
KM Patent Rights, to Exploit and/or have Exploited Antibodies originally
obtained, isolated, identified or derived from such mice (and/or Antibody
Materials related thereto and/or Antibody Products containing such Antibodies)
and as to which (a) Kirin (and/or a Kirin Affiliate(s), as permitted under
Section 8.5.3 hereof) has entered into a written agreement with one or more
Third Party(ies) with respect to such Third Party's(ies') Exploitation of the
Collaboration Product(s) resulting from such project whereby neither Kirin nor
any Kirin Affiliate retains any Substantial Commercialization Rights with
respect to such Collaboration Product(s); or (b) Kirin (and/or a Kirin
Affiliate(s), as permitted under Section 8.5.3 hereof) has entered into a
material transfer or other technology evaluation agreement with one or more
Third Party(ies) (other than an agreement that grants to such Third Party(ies) a
sublicense under a License granted by Medarex to Kirin pursuant to this
Agreement) for the purpose of determining whether such Third Party(ies) desires
to conduct a project that meets the requirements set forth in clause (a) of this
Section; provided, however, that a "Kirin Partner Project" shall not include any
(i) project resulting from a Kirin In-House Project; or (ii) Services Project.
1.128 "Kirin Patent Rights" shall mean any and all Patent Rights that
Kirin Controls during the period of time commencing on December 27, 1999, and
ending on the last day of the Term, including, without limitation, those listed
on Exhibit F, that cover the composition or use of any Collaboration Mice or
that are necessary for the use of any of the Collaboration Mice or the
Exploitation of any Antibody originally obtained, isolated, identified, or
derived from one or more Collaboration Mice, or an Antibody Product containing
such Antibody, but excluding all Excluded Claims, all KM Patent Rights, all
Medarex Patent Rights and additionally excluding all Patent Rights in-licensed
by Kirin from a Third Party after the Effective Date. The Kirin Patent Rights
shall be deemed to include all patent claim(s) within such Patent Rights that
are directed to FcaRII-KO mice and mice produced by breeding any mice with
FcaRII-KO mice, which are not within the KM Patent Rights.
1.129 "Kirin Primary Promotional Area" shall mean countries in Asia.
1.130 "Kirin Reservation License" shall mean each license granted to
Kirin by Medarex pursuant to Section 8.2.3 hereof.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-16-
1.131 "Kirin Reservation Target" shall mean an Antigen (a) that Kirin
has, prior to the Effective Date and in reliance on the Agreement on Essential
Terms for Collaboration, designated as an In-House Target; or (b) with respect
to which Kirin exercises its rights under a Kirin Reservation License pursuant
to and in accordance with Section 4.2.3 hereof; provided, however, that any such
Antigen shall cease to be a Kirin Reservation Target upon the expiration of the
applicable Reservation License Period or termination of the Kirin Reservation
License granted with respect to such Antigen.
1.132 "Kirin Semi-Exclusive Commercial Target" shall mean an Antigen
with respect to which Kirin exercises its rights under a Kirin Antigen
Semi-Exclusive Commercial License pursuant to and in accordance with Section
4.3.2 or 4.3.4 hereof; provided, however, that any such Antigen shall cease to
be a Kirin Semi-Exclusive Commercial Target upon the expiration or termination
of the Kirin Antigen Semi-Exclusive Commercial License granted with respect to
such Antigen.
1.133 "Kirin Target" shall mean any Kirin Reservation Target, Kirin
Non-Exclusive Commercial Target, Kirin Exclusive Commercial Target or Kirin
Semi-Exclusive Commercial Target, as the case may be.
1.134 "Kirin Technology" shall mean the Kirin Patent Rights and the
Kirin Know-How.
1.135 "Kirin Trademarks" shall mean the Trademarks listed in Exhibit
J.
1.136 "KM Know-How" shall mean all Information and Inventions with
respect to which Kirin and/or Medarex, during the period of time commencing on
December 27, 1999, and ending on the last day of the Term, possesses rights,
whether directly or indirectly, whether by ownership, license, contract or
otherwise, to assign or grant a license, sublicense or other right, and which
Information and Inventions are necessary for (a) the use of any mouse containing
both a human immunoglobulin transgene and a human immunoglobulin
transchromosome, but not any mouse containing a human immunoglobulin transgene
but not a human immunoglobulin transchromosome or any mouse containing a human
immunoglobulin transchromosome but not a human immunoglobulin transgene, (b) the
Exploitation of any Antibody derived from a mouse described in clause (a), or
any Antibody Product containing such Antibody, and/or (c) the performance of
activities covered by the KM Patent Rights and are not generally known, but in
each case only to the extent conceived, reduced to practice or otherwise
developed by one or more employees or agents of Medarex, Medarex Affiliates,
Kirin and/or Kirin Affiliates, including, without limitation: (i) biological,
chemical pharmacological, toxicological, pharmaceutical, physical and
analytical, clinical, safety and quality control data and information necessary
to reproduce, raise or use the KM-Mice or Antibodies, cells or nucleic acids
originally obtained, isolated, identified or derived from one or more KM-Mice;
and (ii) assays and methodologies necessary for the use of KM-Mice; provided,
however, that "KM Know-How" excludes any Excluded Know-How and any Information
and Inventions to the extent claimed by the KM Patent Rights.
1.137 "KM-MouseTM" shall mean any mouse with respect to which Medarex
and/or Kirin possess the right, whether directly or indirectly, and whether by
ownership, license, contract or otherwise, to assign or grant a license,
sublicense or other right in and to such mouse, and which mouse contains both
(a) human immunoglobulin transgene nucleic acids; and (b) human immunoglobulin
transchromosome nucleic acids, including, without limitation, any mouse
containing the nucleic acids described in clause (a) and clause (b) of this
Section that is derived by (i) crossbreeding the HuMAb Mouse(R) with a TC
MouseTM, with an HAC MouseTM, and/or with any crossbreed of a TC MouseTM and an
HAC MouseTM, (ii) introducing nucleic acids obtained, isolated, identified, or
derived from one or more HuMAb Mouse(R) into one or more cells obtained from a
TC MouseTM, from an HAC MouseTM, and/or from any crossbreed of a TC MouseTM and
an HAC MouseTM, (iii) introducing nucleic acids
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-17-
obtained, isolated, identified, or derived from one or more TC Mouse(TM), HAC
Mouse(TM), and/or any crossbreed of a TC Mouse(TM) and/or HAC Mouse(TM) into one
or more cells obtained from a HuMAb Mouse(R), (iv) de novo introduction of human
immunoglobulin transgene nucleic acids developed by Medarex or otherwise
developed through use of Medarex Mice into the germline of a mouse, or de novo
introduction of human immunoglobulin transchromosome nucleic acids developed by
Kirin or otherwise developed through use of Kirin Mice into one or more cells of
a mouse, or (v) any other method. "KM-Mouse(TM)" shall also include any mouse
generated by breeding a KM-Mouse(TM) with either any other KM-Mouse(TM) or any
other mouse. For purposes of clarification, no KM-Mouse(TM) shall be deemed to
be a Medarex Mouse or Kirin Mouse, or an Improvement to a Medarex Mouse or a
Kirin Mouse, nor shall any Medarex Mouse or Kirin Mouse be deemed a
KM-Mouse(TM). "KM-Mice" shall mean more than one KM-Mouse(TM).
1.138 "KM Patent Rights" shall mean any and all Patent Rights with
respect to which Medarex and/or Kirin, during the period of time commencing on
December 27, 1999, and ending on the last day of the Term, possess rights,
whether directly or indirectly, whether by ownership, license, contract or
otherwise, to assign or grant a license, sublicense or other right, and which
Patent Rights (a) cover the composition or use of any mouse containing both a
human immunoglobulin transgene and a human immunoglobulin transchromosome, and
that do not cover any mouse containing a human immunoglobulin transgene but not
a human immunoglobulin transchromosome or any mouse containing a human
immunoglobulin transchromosome but not a human immunoglobulin transgene, (b) are
necessary for the use of any mouse described in clause (a) above, or (c) are
necessary for the Exploitation of any Antibody originally obtained, identified,
isolated or derived from any mouse described in clause (a) above, or an Antibody
Product containing such Antibody, but excluding all (i) Excluded Claims; and
(ii) Patent Rights in-licensed by Kirin or Medarex from a Third Party after the
Effective Date. For the avoidance of doubt, patent claims within the Patent
Rights that are specifically directed to FcaRII-KO mice and/or mice produced by
breeding any mice with FcaRII-KO mice, which contain both a human immunoglobulin
transgene and a human immunoglobulin transchromosome shall be deemed within the
KM Patent Rights. A list of the KM Patent Rights known to both Parties as of the
Effective Date is attached as Exhibit G hereto, and the Parties agree to update
such list from time to time to reference any and all other Patent Rights
comprising KM Patent Rights (as defined in the previous two sentences). For the
purpose of this Section 1.138, a "transgene" shall mean a gene (or genes) that
is foreign to the host organism and is inserted and physically integrated into
the host genome DNA, and a "transchromosome" shall mean a chromosome,
chromosomes, a fragment or fragments thereof, or a vector or vectors derived
therefrom, comprising at least one set of telomere and centromere sequences,
which sequences enable autonomous replication in the host cell, and is distinct
from, noncontiguous with, and is not inserted into the host genome DNA.
1.139 "KM Trademarks" shall mean the Trademarks listed in Exhibit K.
In the event that either Party develops additional Trademarks intended for use
in connection with the KM-MouseTM, the Parties shall discuss in good faith
whether to amend Exhibit K to include such Trademarks as KM Trademarks.
1.140 "Know-How" shall mean the Medarex Know-How or the Kirin
Know-How, as the case may be.
1.141 "License" shall mean a Reservation License, an Antigen
Exclusive Commercial License, an Antigen Semi-Exclusive Commercial License, or
an Antigen Non-Exclusive Commercial License, as the case may be.
1.142 "Licensed Trademarks" shall mean in the case of Medarex, the
Medarex Trademarks, and in the case of Kirin, the Kirin Trademarks.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-18-
1.143 "[*]" shall mean with respect to each Antigen that has been
selected by a Party as a Non-Exclusive Commercial Target for the exercise of
rights under an Antigen Non-Exclusive Commercial License, [*] with respect to
which the licensee Party (and/or its sublicensee) has a right to exercise such
License, which [*] is accomplished in accordance with Section 4.4.2 hereof,
where applicable.
1.144 "[*]" shall mean, with reference to each Party's In-House
Target List, [*].
1.145 "Lists" shall mean the Antigen Lists and the Antibody Sequence
List.
1.146 "Losses" shall mean losses, damages, liabilities, costs, and
expenses (including without limitation, reasonable attorneys' fees and expenses)
in connection with any and all suits, investigations, claims or demands.
1.147 "Major Country" shall mean [*].
1.148 "Major European Countries" shall mean [*].
1.149 "Manufacture" and "Manufacturing" shall mean, with respect to a
product or compound, the manufacturing, processing, formulating, packaging,
labeling, storage, and/or quality control testing of such product or compound,
and, with respect to a process, the use of such process in the manufacture of a
product.
1.150 "Marketing Approval" shall mean, with respect to each country,
on a Collaboration Product-by-Collaboration Product basis, approval of an
applicable MAA filed in such country by the relevant Regulatory Authority in
such country; provided, however, that the absence of pricing or reimbursement
approval with respect to a Collaboration Product shall not preclude the
existence of "Marketing Approval" with respect to such Collaboration Product.
1.151 "Marketing Approval Application" or "MAA" shall mean, on a
Collaboration Product-by-Collaboration Product basis, a New Drug Application or
Biologics License Application as required under the U.S. Food, Drug and
Cosmetics Act and the regulations promulgated thereunder, or a comparable filing
in a country other than the United States.
1.152 "Materials" shall mean the Medarex Materials when referring to
materials provided or to be provided by Medarex to Kirin and shall mean the
Kirin Materials when referring to materials provided or to be provided by Kirin
to Medarex.
1.153 "Medarex" shall mean Medarex, Inc. together with GenPharm
International, Inc., its wholly-owned subsidiary.
1.154 "Medarex Affiliate" shall mean an Affiliate of Medarex. For
purposes of this Agreement, neither GenPharm International, Inc. nor Medarex,
Inc. shall be a Medarex Affiliate.
1.155 "Medarex Antigen Exclusive Commercial License" shall mean each
license granted to Medarex by Kirin in Section 8.3.4(a) hereof.
1.156 "Medarex Antigen Non-Exclusive Commercial License" shall mean
each license granted to Medarex by Kirin in Section 8.3.4(b) hereof.
1.157 "Medarex Antigen Semi-Exclusive Commercial License" shall mean
each license granted to Medarex by Kirin in Section 8.3.4(c) hereof.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-19-
1.158 "Medarex Breeding License" shall mean the license granted to
Medarex by Kirin in Section 8.3.1 hereof.
1.159 "Medarex Commercial Target" shall mean a Medarex Exclusive
Commercial Target, Medarex Semi-Exclusive Commercial Target or Medarex
Non-Exclusive Commercial Target, as the case may be.
1.160 "Medarex Designated Exchange Rate" shall mean the exchange rate
published in the ▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇ Edition, or, if not
so available, as otherwise mutually agreed by the Parties in writing.
1.161 "Medarex Evaluation License" shall mean the license granted to
Medarex by Kirin in Section 8.3.2 hereof.
1.162 "Medarex Exclusive Commercial Target" shall mean an Antigen
with respect to which Medarex exercises its rights under a Medarex Antigen
Exclusive Commercial License pursuant to and in accordance with Section 4.3.2,
4.3.3 or 4.3.4(d) hereof; provided, however, that any such Antigen shall cease
to be a Medarex Exclusive Commercial Target upon the expiration or termination
of the Medarex Antigen Exclusive Commercial License granted with respect to such
Antigen.
1.163 "Medarex Improvement" shall mean any Improvement Controlled by
Medarex, but excluding any Improvements for which the Patent Rights or other
intellectual property rights are in-licensed by Medarex from a Third Party after
the Effective Date.
1.164 "Medarex In-House Project" shall mean a research, development
and/or commercialization project that involves the use of Kirin Mice and/or
KM-Mice, and/or a grant of rights (including, without limitation, a license or a
sublicense) under the Kirin Technology and/or KM Patent Rights (whether or not
such project also involves the use of Medarex Mice and/or a grant of rights
(including, without limitation, a license or a sublicense) under the Medarex
Technology), to Exploit and/or have Exploited Antibodies originally obtained,
isolated, identified or derived from such mice (and/or Antibody Materials
related thereto and/or Antibody Products containing such Antibodies) and as to
which (a) substantially all of the research or development effort will come from
Medarex's (and/or any Medarex Affiliate(s)'s) internal research or development
assets and Medarex (and/or any Medarex Affiliate(s)) retains Substantial
Commercialization Rights with respect to the Antibody Product(s) resulting from
such project; (b) Medarex (and/or any Medarex Affiliate(s), as permitted under
Section 8.5.3 hereof) has entered into a written collaborative research,
development and/or commercialization agreement with one or more Medarex
Affiliate(s) or Third Party(ies) with respect to the Exploitation of the
Antibody Product(s) resulting from such project in exchange for Medarex (and/or
a Medarex Affiliate) receiving Substantial Commercialization Rights thereto; or
(c) Medarex (and/or any Medarex Affiliate(s), as permitted under Section 8.5.3
hereof) has entered into a material transfer or other technology evaluation
agreement with one or more Medarex Affiliate(s) or Third Party(ies) (other than
an agreement that grants to such Medarex Affiliate or Third Party(ies) a
sublicense under a License granted by Kirin to Medarex pursuant to this
Agreement) for the purpose of determining whether Medarex (and/or such Medarex
Affiliate(s)) and such Medarex Affiliate(s) or Third Party(ies) desire to
conduct a project that meets the requirements set forth in clause (b) of this
Section; provided, however, that Medarex's (and/or Medarex Affiliate(s)'s) use
of subcontractors for research, development, or Manufacturing activities
relating to such project shall not affect the characterization of a project as a
"Medarex In-House Project" so long as Medarex and/or such Medarex Affiliate
retains Substantial Commercialization Rights with respect to the Antibody
Product(s) resulting from such project; provided, further, that a "Medarex
In-House Project" shall not include any HuMAb Project.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-20-
1.165 "Medarex In-License Agreement" shall mean a license agreement
listed on Exhibit C.
1.166 "Medarex Internal Project" shall mean an existing or future HuMAb
Project of Medarex (and/or a Medarex Affiliate(s)) with respect to which Medarex
(and/or a Medarex Affiliate(s)) retains Substantial Commercialization Rights to
Antibody Products resulting from such project, and which project is not subject
to the terms of any Project Agreement with a Third Party.
1.167 "Medarex Internal Project List" shall mean a list of all Antigens
selected by Medarex (and/or a Medarex Affiliate(s)) for Medarex Internal
Projects, which list Medarex maintains pursuant to Section 2.2.2 hereof.
1.168 "Medarex Know-How" shall mean all Information and Inventions in
the Control of Medarex during the period of time commencing on December 27,
1999, and ending on the last day of the Term, and which Information and
Inventions are necessary for the use of the Medarex Mice, any Antibody derived
from Medarex Mice, any Antibody Product containing such Antibody, and/or the
performance of activities covered by the Medarex Patent Rights and that are not
generally known, but only to the extent conceived, reduced to practice, or
otherwise developed by one or more employees or agents of Medarex, including,
without limitation, (a) biological, chemical, pharmacological, toxicological,
pharmaceutical, physical and analytical, clinical, safety, and quality control
data and information necessary to reproduce, raise, or use the Medarex Mice or
Antibodies, cells, or nucleic acids originally obtained, isolated, identified,
or derived from one or more of the Medarex Mice; (b) assays and methodologies
necessary for the use of the Medarex Mice; provided, however, that "Medarex
Know-How" excludes any Excluded Know-How, KM Know-How and any Information and
Inventions to the extent claimed by the Medarex Patent Rights.
1.169 "Medarex Lists" shall have the meaning set forth in Section 2.1.1
hereof.
1.170 "Medarex Materials" shall mean Medarex Mice and/or KM-Mice
provided or to be provided by or on behalf of Medarex to Kirin or any Kirin
Affiliate pursuant to Section 9.1.1(a) hereof.
1.171 "Medarex Mouse" shall mean any (a) HuMAb Mouse(R); and (b) any
Improvement of a HuMAb Mouse(R); provided, however, that in no event shall any
mouse classified as a TC Mouse(TM), HAC Mouse(TM), KM-Mouse(TM), or an
Improvement of a TC Mouse(TM), HAC Mouse(TM), or KM-Mouse(TM) be deemed to be a
"Medarex Mouse." "Medarex Mice" shall mean more than one Medarex Mouse.
1.172 "Medarex Non-Exclusive Antigen List" shall mean a list
identifying each Antigen (other than an Antigen that is a ROFR Target) with
respect to which Medarex (a) from time to time during the Term exercises its
rights under a Reservation License and/or an Antigen Non-Exclusive Commercial
License in accordance with the terms and conditions of Article IV hereof; (b)
prior to the Effective Date has entered into an agreement that provides for the
grant to a Medarex Affiliate or Third Party of a license or other rights
(including, without limitation, an option or a right of first refusal) in and to
the Medarex Mice, Kirin Mice and/or KM-Mice to raise Antibodies against such
Antigen on an Antigen non-exclusive basis; and (c) prior to or during the Term,
exercises or grants rights on an Antigen non-exclusive basis in connection with
a HuMAb Project.
1.173 "Medarex Non-Exclusive Commercial Target" shall mean an Antigen
with respect to which Medarex exercises its rights under a Medarex Antigen
Non-Exclusive Commercial License pursuant to and in accordance with Section
4.3.2, 4.3.3 or 4.3.4(d) hereof; provided, however, that any such Antigen shall
cease to be a Medarex Non-Exclusive Commercial Target upon the expiration or
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-21-
termination of the Medarex Antigen Non-Exclusive Commercial License granted with
respect to such Antigen.
1.174 "Medarex Partner" shall mean, with respect to a Medarex Partner
Project, the Third Party with which Medarex (and/or a Medarex Affiliate, as
permitted under Section 8.5.3) has entered or enters into a Project Agreement
governing such Medarex Partner Project.
1.175 "Medarex Partner Payment" shall mean consideration of any type
received by Medarex (and/or any Medarex Affiliate(s)) from a Partner in
connection with a Medarex Partner Project, including, without limitation, up
front payments, license fees and milestones, but excluding amounts received in
the form of (a) royalty payments on sales of products; (b) amounts received, at
or below fair market value, for services provided by Medarex (or a Medarex
Affiliate), such as producing Antibodies and Manufacturing Antibody Products or
conducting research; (c) amounts received, at or below fair market value, for
equity in Medarex (or a Medarex Affiliate); (d) equity received from a Partner
in exchange for monetary consideration at or above fair market value; or (e)
amounts received in the form of a loan to Medarex (or a Medarex Affiliate) or
repayment of a loan from Medarex (or a Medarex Affiliate); provided, however,
that Partner Payments shall not include any consideration received by Medarex
(and/or any Medarex Affiliate(s)) from a Medarex Partner in connection with a
Medarex Partner Project to the extent that such consideration was provided in
exchange for access to, the right to immunize, and/or the grant of a license
that covers, Medarex Mice or to the extent such consideration relates to a
product or process that was not developed using Kirin Mice or KM-Mice. For
purposes of this definition, in the event that any such consideration is
received by Medarex (and/or any Medarex Affiliate(s)) from any Medarex Partner
in exchange for the grant by Medarex (or a Medarex Affiliate) to such Medarex
Partner of access, the right to immunize and/or a license, which access is to,
and the right to immunize and/or such license [*] shall constitute a "Medarex
Partner Payment"; provided, however, that in the event Medarex (or a Medarex
Affiliate) grants access to, the right to immunize, or a license that [*] and
the [*]."
1.176 "Medarex Partner Project" shall mean a research, development
and/or commercialization project that involves the use of Kirin Mice and/or
KM-Mice, and/or a grant of rights (including, without limitation, a license or a
sublicense) under the Kirin Technology and/or KM Patent Rights (whether or not
such project also involves the use of Medarex Mice and/or a grant of rights
(including, without limitation, a license or a sublicense) under the Medarex
Technology), to Exploit and/or have Exploited Antibodies originally obtained,
isolated, identified or derived from such mice (and/or Antibody Materials
related thereto and/or Antibody Products containing such Antibodies) and as to
which (a) Medarex (and/or a Medarex Affiliate(s), as permitted under Section
8.5.3 hereof) has entered into a written agreement with one or more Third
Party(ies) with respect to such Third Party's(ies') Exploitation of the
Collaboration Product(s) resulting from such project whereby neither Medarex nor
any Medarex Affiliate retains any Substantial Commercialization Rights with
respect to such Collaboration Product(s); or (b) Medarex (and/or a Medarex
Affiliate(s), as permitted under Section 8.5.3 hereof) has entered into a
material transfer or other technology evaluation agreement with one or more
Third Party(ies) (other than an agreement that grants to such Third Party(ies) a
sublicense under a License granted by Kirin to Medarex pursuant to this
Agreement) for the purpose of determining whether such Third Party(ies) desires
to conduct a project that meets the requirements set forth in clause (a) of this
Section; provided, however, a "Medarex Partner Project" shall not include any
(i) project resulting from a Medarex In-House Project or HuMAb License Project;
(ii) HuMAb Project; or (iii) Services Project.
1.177 "Medarex Patent Rights" shall mean any and all Patent Rights that
Medarex Controls as of December 27, 1999, and during the Term, including,
without limitation, those listed on Exhibit H, in each case that cover the
composition or use of any Collaboration Mice, or that are necessary for the
Exploitation of any of the Collaboration Mice, or any Antibody originally
obtained, isolated, identified, or
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-22-
derived from one or more of the Collaboration Mice, or an Antibody Product
containing such Antibody, but excluding all Excluded Claims, all Kirin Patent
Rights, all KM Patent Rights and all Patent Rights in-licensed by Medarex from a
Third Party after the Effective Date.
1.178 "Medarex Primary Promotional Area" shall mean countries outside
of Asia.
1.179 "Medarex Reservation License" shall mean each license granted to
Medarex by Kirin in Section 8.3.3 hereof.
1.180 "Medarex Reservation Target" shall mean an Antigen with respect
to which Medarex exercises its rights under a Medarex Reservation License
pursuant to and in accordance with Section 4.2.2 hereof; provided, however, that
any such Antigen shall cease to be a Medarex Reservation Target upon the
expiration of the applicable Reservation License Period or termination of the
Medarex Reservation License granted with respect to such Antigen.
1.181 "Medarex Restricted Antigen List" shall mean a list identifying
each Antigen with respect to which Medarex (a) prior to December 27, 1999, has
entered into an agreement that provides for the grant to an Affiliate or a Third
Party of a license or other rights (including, without limitation, an option or
a right of first refusal) in and to the Medarex Mice, Kirin Mice and/or KM-Mice
to raise Antibodies against such Antigen on an Antigen-exclusive basis; or (b)
on or after December 27, 1999, has entered or enters into an agreement with an
Affiliate or Third Party that provides for the grant to an Affiliate or Third
Party of a license or other rights (including, without limitation, an option or
a right of refusal) in connection with a HuMAb Project (other than a Medarex
Internal Project) to raise Antibodies against such Antigen on an
Antigen-exclusive basis.
1.182 "Medarex Semi-Exclusive Commercial Target" shall mean an Antigen
with respect to which Medarex exercises its rights under a Medarex Antigen
Semi-Exclusive Commercial License pursuant to and in accordance with Section
4.3.2 or 4.3.3 hereof; provided, however, that any such Antigen shall cease to
be a Medarex Semi-Exclusive Commercial Target upon the expiration or termination
of the Medarex Antigen Semi-Exclusive Commercial License granted with respect to
such Antigen.
1.183 "Medarex Target" shall mean any Medarex Reservation Target,
Medarex Non-Exclusive Commercial Target, Medarex Exclusive Commercial Target, or
Medarex Semi-Exclusive Commercial Target, as the case may be.
1.184 "Medarex Technology" shall mean the Medarex Patent Rights and the
Medarex Know-How.
1.185 "Medarex Trademarks" shall mean the Trademarks listed in Exhibit
L.
1.186 "Mice Material" shall mean, with respect to any Collaboration
Mice, parts or derivatives of such mice, including hybridomas, cells or other
biological materials, nucleic acids (including DNA, RNA, and complementary and
reverse complementary nucleic acids thereto, whether coding or noncoding and
whether intact or a fragment), and any replicates or modifications of any of the
foregoing, but excluding any Antibody(ies) and any Antibody Material(s).
1.187 "Milestone Event" shall have the meaning set forth in Section
10.1.3 hereof.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-23-
1.188 "[*] Partner Payments" shall mean the [*] amounts payable by a
Party to the other Party in connection with Partner Payments received by the
first Party from a Partner as calculated in accordance with Section 10.7.5
hereof.
1.189 "[*]" shall have the meaning set forth in Section 10.1.3 hereof.
1.190 "MRC Agreement" shall mean that certain License Agreement entered
into by the Medical Research Council, Agricultural and Food Research Council
Institute of Animal Physiology and Genetics Research of Babraham Hall, and ▇▇.
▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, on the one hand, (collectively, the "MRC Parties") and
GenPharm International, Inc., on the other hand, effective October 1, 1993, as
amended on August 12, 1994, and on April 19, 2002, and as amended further from
time to time, which further amendment(s) is consistent with Section 16.2.2
hereof. "MRC" shall mean the MRC Parties, or their respective assign(s) or
successor(s)-in-interest under the MRC Agreement, as applicable.
1.191 "Net Sales" shall mean the gross amount invoiced by a Party, its
Affiliates, licensees and sublicensees to Third Parties for sales of
Collaboration Products, less deductions for (a) normal and customary trade,
quantity and cash discounts and sales returns and allowances (other than
allowances for doubtful accounts), including, without limitation, (i) those
granted on account of price adjustments, billing errors, rejected goods, damaged
goods, returns and rebates, (ii) administrative and other fees and
reimbursements and similar payments directly related to the sale or delivery of
Collaboration Product(s) paid to wholesalers and other distributors, buying
groups, pharmacy benefit management organizations, health care insurance
carriers and other institutions, (iii) allowances, rebates and fees directly
related to the sale or delivery of Collaboration Product(s) paid to distributors
and (iv) chargebacks; (b) freight, postage, shipping and insurance costs to the
extent that such items are included in the gross amount invoiced; (c) customs
and excise duties and other duties related to the sales to the extent that such
items are included in the gross amount invoiced; (d) rebates and similar
payments made with respect to sales paid for or reimbursed by any governmental
or regulatory authority such as, by way of illustration and not in limitation of
the Parties' rights hereunder, Federal or state Medicaid, Medicare or similar
state program or equivalent foreign governmental program; (e) sales and other
taxes and duties directly related to the sale or delivery of Collaboration
Product(s) (but not including taxes assessed against the income derived from
such sale); (f) distribution costs and expenses to the extent that such items
are included in the gross amount invoiced; and (g) any such invoiced amounts
that are not collected by the Parties or their Affiliates, licensees or
sublicensees after the Party, Affiliate, licensee or sublicensee has made
commercially reasonable efforts to collect the same; provided, however, that an
amount shall be deducted only once regardless of how many categories may apply
to it. Any of the deductions listed in (a) through (f) above that involves a
payment by a Party or its Affiliates, licensees or sublicensees shall be taken
as a deduction in the Calendar Quarter in which the payment is accrued by such
entity. Deductions pursuant to clause (g) above shall be taken in the Calendar
Quarter in which such sales are no longer recorded as a receivable. For purposes
of determining Net Sales, the Collaboration Product(s) shall be deemed to be
sold when invoiced and a "sale" shall not include transfers or dispositions for
charitable, promotional, pre-clinical research, clinical-trial, regulatory or
governmental purposes, in any such case, to the extent not for fair market
value. For purposes of calculating Net Sales of Collaboration Products, sales
between or among the Parties or their Affiliates, licensees, or sublicensees
shall be excluded from the computation of Net Sales, but sales by a Party or its
Affiliates, licensees, or sublicensees to other Third Parties shall be included
in the computation of Net Sales.
In the event that a Collaboration Product is sold in any country in the form of
a combination product containing one or more active ingredients in addition to
any Collaboration Antibody contained in such product, the Parties shall
negotiate in good faith to determine what portion of the net sales of such
combination product in such country shall be treated as "Net Sales" under this
Agreement, which
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-24-
determination shall be based on the value added by such Collaboration Antibody,
compared to the value added by such other active ingredients, to the invoice
price of such combination.
1.192 "Non-Contracting Party" shall mean, with respect to any Project
Agreement governing a Partner Project entered into or contemplated by a
Contracting Party, the Party other than the Contracting Party.
1.193 "Non-Designating Party" shall mean, with respect to any In-House
Target, the Party other than the Party that designates such Target on its
In-House Target List.
1.194 "Non-Exclusive Antigen List" shall mean either the Kirin
Non-Exclusive Antigen List or the Medarex Non-Exclusive Antigen List, as the
case may be.
1.195 "Non-Exclusive Commercial Target" shall mean a Kirin
Non-Exclusive Commercial Target or a Medarex Non-Exclusive Commercial Target, as
the case may be.
1.196 "Non-Paying Party" shall mean, with respect to any Project
Agreement entered into by a Party that governs a Partner Project, the Party
other than the Party that is required to make [*] Partner Payments pursuant to
Section 10.7.5 hereof.
1.197 "Non-Requesting Party" shall mean the Party to which the
Requesting Party may make requests pursuant to Section 6.4 hereof.
1.198 "Non-Responsible Party" shall mean (a) Kirin in the case of [*]
to prosecute and/or maintain pursuant to Section 12.2.1(b) hereof; (b) Medarex
in the case of any [*] to prosecute and/or maintain pursuant to Section
12.2.2(b) hereof; and (c) in the case of any KM Patent Rights and KM Trademarks,
the Party other than the Party that has the first right of preparation, filing,
prosecution and maintenance of such KM Patent Rights and registrations of such
KM Trademarks pursuant to Section 12.2.4(a) hereof.
1.199 "Non-Terminating Party" shall have the meaning set forth in
Section 8.7.1 hereof.
1.200 "Notice of Rights Preserving Excuse" shall have the meaning set
forth in Section 8.11.3 hereof.
1.201 "Obligor Party" shall have the meaning set forth in Section
8.11.1 hereof.
1.202 "Opt-In Offer" shall mean, with respect to a Target added by the
Designating Party to its In-House Project List, an offer made in good faith by
the Designating Party to the Non-Designating Party which if accepted would
entitle the Non-Designating Party to obtain Commercialization Rights with
respect to any Collaboration Product(s) containing a Collaboration Antibody(ies)
developed, in whole or in part, by the Designating Party (and/or its In-House
Collaborator) against a particular Target, which in the case of Medarex as the
Non-Designating Party shall include [*], and in the case of Kirin as the
Non-Designating Party shall include [*].
1.203 "Opt-Out" shall mean an election made by a Party, its Affiliate
and/or its In-House Collaborator(s) pursuant to which election the electing
Person relinquishes its rights to pursue research, development, and
commercialization activities with respect to one or more Antibodies raised
against and with affinity for a Target designated by such Party on its In-House
Target List.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-25-
1.204 "Original Antibody Inquiry Response Period" shall have the meaning
set forth in Section 3.5.2(a) hereof.
1.205 "Original Antigen Inquiry Response Period" shall have the meaning set
forth in Section 3.3.1(d)(i) hereof.
1.206 "Partner" shall mean either a Medarex Partner or a Kirin Partner, as
the case may be.
1.207 "Partner Payment" shall mean a Medarex Partner Payment received by
Medarex (or a Medarex Affiliate) from a Medarex Partner or a Kirin Partner
Payment received by Kirin (or a Kirin Affiliate) from a Kirin Partner, as the
case may be.
1.208 "Partner Project" shall mean a Medarex Partner Project or a Kirin
Partner Project, as the case may be.
1.209 "Partner Project Status Change" shall mean a change that a Party
intends to make or actually makes in accordance with Section 7.2 hereof in its
rights to an Antibody(ies) raised against and with affinity for a particular
Antigen (and Antibody Materials and Antibody Products relating thereto) in
connection with a Partner Project with the result that such project would no
longer satisfy the definition of a Partner Project and would instead qualify as
an In-House Project hereunder, which change shall include, without limitation,
the exercise of an option held by such Party for Substantial Commercialization
Rights.
1.210 "Partner Project Status Change Date" shall mean the date on which a
Party effects a Partner Project Status Change in accordance with Section 7.2
hereof.
1.211 "Partner Royalties" shall mean any royalty payments received by
Medarex (and/or a Medarex Affiliate) or by Kirin (and/or a Kirin Affiliate), as
the case may be, from a Partner in connection with a Partner Project; provided,
however, that with respect to Medarex[*].
1.212 "Patent Rights" shall mean (a) all patents and patent applications
(and any claims contained therein), wherever filed; (b) all patents and patent
applications arising out of the patent applications and/or patents described in
clause (a), including all substitutions, divisions, continuations,
continuations-in-part, reissues, renewals, registrations, confirmations,
re-examinations, extensions, supplementary protection certificates and the like,
and any provisional applications, of any such patents or patent application; and
(c) any foreign or international equivalent of any of the foregoing (including,
without limitation, a PCT application).
1.213 "Paying Party" shall mean, with respect to any Project Agreement
entered into by a Party governing a Partner Project, the Party that is required
to make [*] Partner Payments to the other Party pursuant to Section 10.7.5
hereof.
1.214 "Person" shall mean an individual, sole proprietorship, partnership,
limited partnership, limited liability partnership, corporation, limited
liability company, business trust, joint stock company, trust, unincorporated
association, joint venture or other similar entity or organization, including a
government or political subdivision, department or agency of a government.
1.215 "Phase I Trial" shall mean a human clinical trial, the principal
purpose of which is a preliminary determination of safety in healthy individuals
or patients as required in 21 C.F.R. ss. 312, or a similar clinical study
prescribed by the Regulatory Authorities in a country other than the United
States. A Phase I Clinical Trial shall be deemed to have commenced when the
first subject in the study has been
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-26-
enrolled. For the avoidance of doubt, a combined Phase I / Phase II Trial shall
be deemed a Phase I Trial for the purposes of this Agreement.
1.216 "Phase II Trial" shall mean a human clinical trial for which a
primary endpoint is a preliminary determination of efficacy or dose ranges in
patients with the disease being studied as required in 21 C.F.R. ss. 312, or a
similar clinical study prescribed by the Regulatory Authorities in a country
other than the United States. A Pivotal Study shall automatically be deemed to
have reached Phase II status. A Phase II Trial shall be deemed to have commenced
when the first subject in the study has been enrolled.
1.217 "Phase III Trial" shall mean a human clinical trial, the principal
purpose of which is to establish safety and efficacy in patients with the
disease being studied as required in 21 C.F.R. ss. 312, or similar clinical
study prescribed by the Regulatory Authorities in a country other than the
United States. A Phase III Trial shall also include any other human clinical
trial intended as a Pivotal Study, whether or not such study is a traditional
Phase III Trial. A Phase III Trial shall be deemed to have commenced when the
first patient has been enrolled in a Pivotal Study.
1.218 "Pivotal Study" shall mean any well-controlled study intended to
provide the substantial evidence of efficacy necessary to support the filing of
an approvable MAA (such as a combined Phase II Trial / Phase III Trial, or any
Phase III Trial in lieu of a Phase II Trial).
1.219 "Primary Promotional Area" shall mean the Medarex Primary Promotional
Area or Kirin Primary Promotional Area, as the case may be.
1.220 "Production Process Development" shall mean the development of
processes and technology for the identification, isolation, selection,
modification, production, purification, evaluation, characterization,
stabilization, vialing and distribution, or release of an Antibody, Antibody
Materials, or Antibody Product, including, without limitation, hybridoma fusion
technology, phage display technology, isolation of variable region genes,
modification of antibody variable and/or constant regions, development of
antibody expression vectors, development of recombinant cells for antibody
expression, development of antibody purification processes, and development of
antibody stabilization processes or formulations.
1.221 "Production Process Know-How" shall mean any Information and
Inventions of a Party with respect to the Production Process Development or the
Manufacture of Antibody(ies), Antibody Material(s), or Antibody Product(s), but
excluding any Information and Inventions to the extent covered or claimed by the
Production Process Patents.
1.222 "Production Process Patents" shall mean any patent claims contained
in Patent Rights to the extent that they claim or cover the Production Process
Development or the Manufacture of an Antibody(ies), Antibody Material(s), or
Antibody Product(s).
1.223 "Production Process Technology" shall mean any Production Process
Know-How and Production Process Patents.
1.224 "Project Agreement" shall mean any agreement entered into between a
Party (and/or an Affiliate(s) of such Party, as permitted under Section 8.5.3
hereof to the extent applicable) and one or more of such Party's Affiliate(s) or
Third Party(ies) that governs one or more Partner Projects and/or In-House
Projects (or, with respect to Medarex, HuMAb Collaboration Projects and/or HuMAb
License Projects), but excluding any agreement between a Party (and/or its
Affiliate) and a Third Party that is entered into solely in connection with the
grant by a Third Party to the Party (and/or its Affiliate) of a
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-27-
license or other rights with respect to intellectual property that falls within
the definition(s) of Excluded Claims and/or Excluded Know-How.
1.225 "Reagent" shall mean an Antibody Product used in an assay or test to
detect, measure, examine, analyze or produce substances for research (i.e.,
non-therapeutic, non-prophylactic and non-diagnostic) purposes, but excluding
any In Vivo Therapeutic Application, Ex Vivo Therapeutic Application, or
Diagnostic Application.
1.226 "Receiving Party" shall have the meaning set forth in Section 8.11.1
hereof.
1.227 "Reconciliation Amount" shall have the meaning set forth in Section
10.1.3 hereof.
1.228 "Refusal Rights" shall mean a right of refusal, offer or negotiation
(but excluding an option), in each case to obtain an exclusive, worldwide
license or other exclusive rights relating to a particular Antigen.
1.229 "Regulatory Approval" shall mean any and all approvals (including
pricing and reimbursement approvals), licenses, registrations or authorizations
of any Regulatory Authority, necessary for the Exploitation of a Collaboration
Product in a country, including any (a) approval of such Collaboration Product,
including without limitation any INDs, New Drug Applications, Biologic License
Applications (in each case as required under the U.S. Food, Drug and Cosmetic
Act, as amended, and the regulations promulgated thereunder), and supplements
and amendments thereto; (b) pre- and post-approval marketing authorizations
(including any prerequisite Manufacturing approval or authorization related
thereto); and (c) labeling approval.
1.230 "Regulatory Authority" shall mean the U.S. Food and Drug
Administration and any applicable supra-national, federal, national, regional,
state, provincial or local regulatory agencies, departments, bureaus,
commissions, councils or other government entities equivalent thereto.
1.231 "[*]" shall mean a Collaboration Antibody that is raised against and
with affinity for an Antigen that is the subject of a Commercial License granted
by a Party to the other Party and that the licensee Party desires to [*]
pursuant to Section 4.4.2 hereof.
1.232 "[*]" shall mean the date as of which a [*] is deemed to be [*] for a
Commercial License pursuant to Section 4.4.2(c) hereof.
1.233 "[*]" shall have the meaning set forth in Section 8.11.3 hereof.
1.234 "Requesting Party" shall mean the Party that has a right to make
requests to the other Party and actually makes such requests pursuant to Section
6.4 hereof.
1.235 "Reservation License" shall mean a Kirin Reservation License or a
Medarex Reservation License, as the case may be.
1.236 "Reservation License Fee" shall mean the amount, if any, payable by a
Party to the other Party pursuant to Sections 10.2.1 and 10.2.2 hereof, as
applicable.
1.237 "Reservation License Period" shall mean, on a Reservation
Target-by-Reservation Target basis, the period commencing on the date that the
Party exercises its rights under a Reservation License with respect to a
particular Reservation Target pursuant to Section 4.2.2 or 4.2.3 hereof, as
applicable, and ending on the [*] months after such date, or in the case of a
renewal of a Reservation License pursuant to
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-28-
Section 4.2.4 hereof, [*] months after the date on which such License is
renewed, as the case may be; or (b) the date on which such Party exercises its
rights under a Commercial License with respect to such Reservation Target
pursuant to Section 4.3 hereof; provided, however, that with respect to any
Reservation License that Medarex is deemed to grant to Kirin pursuant to Section
8.8.3 hereof, the "Reservation License Period" shall be the period set forth in
Section 8.8.3 hereof with respect to each such Reservation License.
1.238 "Reservation Target" shall mean a Kirin Reservation Target or a
Medarex Reservation Target, as the case may be.
1.239 "Responsible Party" shall mean (a) Medarex in the case of [*] to
prosecute and/or maintain pursuant to Section 12.2.1(b) hereof; (b) Kirin in the
case of [*] to prosecute and/or maintain pursuant to Section 12.2.2(b) hereof;
and (c) in the case of any KM Patent Rights and KM Trademarks, the Party that
has the first right of preparation, filing, prosecution and maintenance of KM
Patent Rights and registrations of KM Trademarks pursuant to Section 12.2.4(a)
hereof.
1.240 "Rights Preserving Excuse" shall have the meaning set forth in
Section 8.11.1 hereof.
1.241 "ROFR Party" shall mean either (a) a Third Party to which a Party has
granted rights to a ROFR Target prior to the Effective Date (regardless of
whether an Antigen has been selected by such Third Party prior to the Effective
Date as an Antigen for which such Third Party shall have Refusal Rights); or (b)
Kirin, with respect to Antigens that Kirin has selected as In-House Targets
prior to the Effective Date, as confirmed by the Shared Exclusive Antigen List.
1.242 "ROFR Target" shall mean (a) an Antigen that is or becomes the
subject of Refusal Rights which a Party has granted or is contractually required
to grant to a Third Party as a result of an agreement between such Party (or any
of its Affiliates) and a Third Party that was entered into prior to the
Effective Date (regardless of whether the Antigen(s) with respect to which the
Third Party shall have Refusal Rights is selected by such Third Party prior to
the Effective Date), without giving effect to any renewal, extension, revision,
amendment or other modification to such agreement entered into after the
Effective Date that provides to the Third Party (i) a right to designate an
additional number of Antigens to which such Third Party shall have Refusal
Rights; or (ii) that provides for any extension of time for such Third Party to
exercise its Refusal Rights (other than any extension provided for in such
agreement as it exists as of the Effective Date), or (b) an Antigen that Kirin
has selected as an In-House Target prior to the Effective Date, as confirmed by
the Shared Exclusive Antigen List; provided, however, that in the case of any
Antigen selected by Kirin as an In-House Target prior to the Effective Date,
such Antigen shall be a ROFR Target only for the period beginning on the
Designation Date for such Antigen and ending on the [*] of such date, or such
earlier date as such ROFR Rights are terminated in accordance with Section
4.3.3(e) hereof, at which time such ROFR Target shall remain a Kirin Reservation
Target until the end of Reservation License Period or until Kirin abandons such
Kirin Reservation Target pursuant to Section 5.4 hereof.
1.243 "SEC" shall mean the United States Securities and Exchange
Commission.
1.244 "Selected Antibody" shall mean a Collaboration Antibody [*] for a
Commercial License pursuant to Section 4.4.2(a)(ii), 4.4.2(b)(ii), or 4.4.2(c)
hereof, as applicable; provided, however, that in the case of any Special
Licensee, for purposes of this Agreement, including for purposes of any license
or other rights granted to Medarex by Kirin in this Agreement, "Selected
Antibody" shall mean each antibody with respect to which the Special Licensee
has a license, option, or right of refusal or negotiation to acquire a license
under the Special License, subject to the terms and conditions of the Special
License.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-29-
1.245 "[*]" shall have the meaning set forth in Section 2.3.6(a) hereof.
1.246 "Semi-Exclusive Commercial Target" shall mean a Kirin Semi-Exclusive
Commercial Target or a Medarex Semi-Exclusive Commercial Target, as the case may
be.
1.247 "Services Project" shall mean a project conducted pursuant to an
agreement between a Party (and/or its Affiliate) and an Affiliate of such Party
or a Third Party, which agreement provides for the use of any Collaboration Mice
and/or the grant of rights under any Technology and/or KM Patent Rights, solely
in connection with the performance of contract services by such Affiliate or
Third Party on behalf of such Party (and/or its Affiliate), which services
include or consist of breeding or immunizing any Collaboration Mice, preclinical
studies or clinical trial programs with respect to Antibodies or Antibody
Products, Manufacturing services and/or Manufacturing process development
services.
1.248 "Shared Exclusive Antigen List" shall mean a list identifying each
Antigen, other than an Antigen included on the Medarex Restricted Antigen List,
that (a) has been selected or is selected in accordance with the terms and
conditions of Article IV hereof (i) by Medarex on an Antigen-exclusive basis in
connection with a Medarex Internal Project; (ii) by Medarex or Kirin for the
exercise of its rights under an Antigen Exclusive Commercial License or an
Antigen Semi-Exclusive Commercial License; or (b) is a ROFR Target.
1.249 "[*]" shall have the meaning set forth in Section [*] hereof.
1.250 "Special Licensees" shall mean those certain licensees of Medarex
that hold Special Licenses, as further identified in a writing delivered by
Medarex to Kirin with respect to this Section 1.250 on the date of execution of
this Agreement, which licensees are sometimes referred to herein individually as
"Special Licensee No. 1", "Special Licensee No. 2", "Special Licensee No. 3" and
"Special Licensee No. 4".
1.251 "Special Licenses" shall mean those license agreements entered into
between Medarex and one or more Third Parties, as identified in a writing
delivered by Medarex to Kirin with respect to this Secton 1.251 on the date of
execution of this Agreement, which licenses are sometimes referred to herein
individually as "Special License No. 1", "Special License No. 2", "Special
License No. 3" and "Special License No. 4"; provided, however, that in no event
shall any renewal, extension, revision, amendment or other modification thereof
entered into after the Effective Date, to the extent that such renewal,
extension, revision, amendment, or other modification provides to the Special
Licensee any additional rights to access or use for research, development, or
commercial purposes the Kirin Mice, KM-Mice, the Kirin Technology, or KM Patent
Rights, be deemed to be a Special License for any purpose under this Agreement;
provided, further, that for purposes of any payment obligations owed by Medarex
to Kirin pursuant to Sections 10.2.2, 10.3.1(b), and 10.7.4 hereof, the special
financial terms accorded to Special Licenses under such Sections shall be deemed
not to apply to any renewal, extension, revision, amendment or other
modification made to a Special License at any time on or after December 27,
1999, to the extent that any such renewal, extension, revision, amendment, or
other modification provides to the Special Licensee, in addition to the rights
granted to the Special Licensee prior to such date, any rights to access or use
for research, development and/or commercial purposes any Kirin Mice, KM Mice,
Kirin Technology or KM Patent Rights. By way of an example and without
limitation, if Medarex grants to a Special Licensee, pursuant to a Special
License entered into prior to December 27, 1999, a right to obtain five (5)
Antigen-exclusive commercial licenses under Kirin Technology and/or KM Patent
Rights, and if any renewal, extension, revision, amendment or other modification
to the applicable Special License on or after December 27, 1999, grants to the
Special Licensee a right to obtain ten (10) Antigen-exclusive commercial
licenses under such technology, for purposes of any payment obligation owed by
Medarex to
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-30-
Kirin pursuant to Sections 10.2.2, 10.3.1(b), and 10.7.4 hereof, the special
financial terms accorded to such Special License under such Sections shall be
accorded to the renewed, extended, revised, amended or modified Special License
only with respect to the five (5) such licenses originally granted under the
form of Special License entered into with such Special Licensee prior to
December 27, 1999. Notwithstanding the foregoing, any renewal, extension,
revision, amendment, or other modification of a Special License entered into by
Medarex and a Special Licensee in connection with negotiations conducted by
Medarex at Kirin's request pursuant to a separate writing signed by both
Parties, shall not affect the status of such agreement as a Special License;
provided, however, that any such renewal, extension, revision, amendment or
other modification beyond that which has been requested by Kirin and that is
inconsistent with the terms of this Agreement shall be subject to Kirin's prior
written consent, which consent shall not be unreasonably withheld.
1.252 "Special Relationship" shall mean, with respect to a Party and a
Third Party, either that (a) as of December 27, 1999, there was in force a
Transfer Agreement between such Party (or its Affiliate(s)) and such Third
Party, and that as of such date there was not in force a Transfer Agreement
between such Third Party and the other Party hereto; (b) as of December 27,
1999, there was in force a Transfer Agreement between such Party (or its
Affiliate(s)) and such Third Party, and as of such date there was also in force
a Transfer Agreement between such Third Party and the other Party hereto (or its
Affiliate(s)), but the Transfer Agreement of the first Party (or its
Affiliate(s)) predates the Transfer Agreement of the other Party (or its
Affiliate(s)), and, if Kirin (or its Affiliate(s)) is such first Party, Medarex
(or its Affiliate(s)) did not have a Transfer Agreement with such Third Party
with respect to Antibodies as of December 27, 1999; or (c) a Transfer Agreement
had been in force between such Party (or its Affiliate(s)) and such Third Party
within three (3) years prior to December 27, 1999, and there was not in force,
and had not been in force within two (2) years prior to December 27, 1999, a
Transfer Agreement between such Third Party and the other Party hereto (or its
Affiliate(s)).
1.253 "Substantial Commercialization Rights" shall mean commercialization
rights with respect to an Antibody Product that shall include at least rights
(including, without limitation, a license but not [*]) to engage in marketing or
promotional activities relating to such Antibody Product in at least [*] and to
share in at least [*] of the economic benefit derived from such Antibody Product
in such [*].
1.254 "[*]" shall have the meaning set forth in Section 10.7.5(c)(ii)
hereof.
1.255 "Target" shall mean a Medarex Target or a Kirin Target, as the case
may be.
1.256 "TC Mouse(TM)" shall mean any immunizable transchromosomic mouse
developed by or for Kirin and/or Kirin Affiliates that contains one or more
human chromosomes or fragments thereof that include centromere and telomere
sequences that provide for replication outside of mouse chromosomes, and which
chromosome(s) or fragment(s) comprises an unrearranged human immunoglobulin
locus; provided, however that in no event shall a Medarex Mouse, a HAC Mouse(TM)
or a KM-Mouse(TM) be considered a "TC Mouse(TM)" for any purpose.
1.257 "Technology" shall mean Medarex Technology or Kirin Technology, as
the case may be.
1.258 "Term" shall mean the period beginning on the Effective Date and
continuing until December 31, 2014, unless terminated at an earlier date in
accordance with the terms and conditions set forth in Article XIV hereof.
1.259 "Terminating Party" shall have the meaning set forth in Section
8.7.1 hereof.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-31-
1.260 "Third Party" shall mean any Person other than Medarex, Kirin, or
their respective Affiliates.
1.261 "Third Party Claim" shall mean claims of any Third Party subject to
indemnification pursuant to Section 15.1 or 15.2.1 hereof.
1.262 "Third Party IP Infringement Suit" shall mean a trademark, patent or
other infringement suit against Kirin, Medarex or any of their respective
Affiliates during the Term of this Agreement alleging that the use of a Licensed
Trademark or Collaboration Mouse, the Exploitation of a Collaboration Antibody,
Collaboration Product or a method or process relating to the use and/or
Exploitation of any of the foregoing, infringes one or more trademarks, patents
or other intellectual property rights held by such Third Party.
1.263 "[*]" shall mean a [*] with which Medarex and Kirin enter into a [*]
Agreement.
1.264 "[*]" shall mean an agreement entered into by Medarex and Kirin, on
the one hand, and the [*], on the other hand, pursuant to Section 2.3.6 hereof.
1.265 "Trademark" shall include any word, name, symbol, color,
designation, or device or any combination thereof, including any trademark,
trade dress, service ▇▇▇▇, service name, brand ▇▇▇▇, trade name, brand name,
logo, or business symbol.
1.266 "Transfer Agreement" shall mean a binding written agreement that
effects a present transfer of significant commercial rights, including, without
limitation, a research and development agreement or license agreement. For
purposes of clarification, a Transfer Agreement shall not include a material
transfer or an evaluation agreement, unless such agreement effects the present
transfer of significant commercial rights.
1.267 "Valid Claim" shall mean, with respect to a particular country, a
claim of an issued and unexpired patent in such country that (a) has not been
revoked or held unenforceable or invalid by a decision of a court or
governmental agency of competent jurisdiction from which no appeal can be taken
or has been taken within the time allowed for appeal; (b) has not been
abandoned, disclaimed, denied, or admitted to be invalid or unenforceable
through reissue or disclaimer or otherwise in such country; and (c) is not
within the Excluded Claims.
ARTICLE II
ANTIGEN AND ANTIBODY LISTS AND RELATED LISTING PROCEDURES
AND DISCLOSURE REQUIREMENTS
2.1 Antibody Sequence List and Antigen Lists Maintained by Each Party.
2.1.1 By Medarex. Medarex shall establish and maintain during the
Term and in accordance with Section 2.3 hereof the following three (3) lists,
collectively referred to as the "Medarex Lists":
(i) Medarex Restricted Antigen List,
(ii) Medarex Non-Exclusive Antigen List, and
(iii) Antibody Sequence List.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-32-
Medarex shall also establish and maintain the Shared Exclusive Antigen List.
Kirin shall assist Medarex in establishing and maintaining the Shared Exclusive
Antigen List and the Antibody Sequence List by providing to Medarex the written
notices required pursuant to Section 2.3.2(a)(ii) hereof.
2.1.2 By Kirin. Kirin shall establish and maintain during the Term
and in accordance with Section 2.3 hereof the Kirin Non-Exclusive Antigen List.
2.2 In-House Target Lists and Medarex Internal Project List.
2.2.1 In House Targets. For purposes of Article V hereof, each Party
shall prepare and maintain during the Term an In-House Target List at all times
in current form, and provide to the other Party a true, correct and complete
copy thereof within [*] business days after the Effective Date, and revised
versions thereof [*] in which any amendment or other change is made thereto,
including the addition or removal of any Target therefrom in accordance with
Article V hereof.
2.2.2 Medarex Internal Project List. Medarex shall establish and
provide to Kirin a Medarex Internal Project List within [*] business days after
the Effective Date, and shall provide to Kirin during the Term an update to the
list [*] in which any amendment or other change is made thereto.
2.3 Listing Procedures. With respect to each list that a Party is required
to establish and maintain pursuant to Section 2.1.1 or 2.1.2 hereof, as
applicable, the Party shall implement and comply with the following procedures:
2.3.1 Description of Antigens and Antibodies.
(a) Antigens. In the event that a Party lists an Antigen in an
Antigen List, an In-House List, or, in the case of Medarex, the Medarex Internal
Project List, the listing Party shall comply with the following listing
requirements, as applicable:
(i) For purposes of a listing by Medarex in the Shared
Exclusive Antigen List of any Antigen with respect to which Kirin (on behalf of
itself, an Affiliate, Partner or In-House Collaborator) has exercised rights
under a Kirin Exclusive Commercial License or a Kirin Semi-Exclusive Commercial
License, Medarex shall describe each such Antigen [*] contained in the [*]
delivered [*], as such information is more fully described in Section [*]
hereof.
(ii) For purposes of a listing by Medarex in the Medarex
Restricted Antigen List, Medarex Non-Exclusive Antigen List and Shared Exclusive
Antigen List of any Antigen with respect to which rights have been granted by
Medarex to a Third Party (other than any of Kirin's In-House Collaborators or
Partners), each Antigen shall be described [*], which description shall include
the following information to the extent that such information is [*] to the
Third Party and [*] such Third Party to Medarex at the time that such Third
Party provides to Medarex a written inquiry regarding the availability of such
Antigen: the Antigen's [*], other [*], the [*], equivalent [*] from other [*],
and the [*] and/or the [*] therefor; provided, however, that if there is [*] or
equivalent [*] from other [*] with respect to such Antigen, such Antigen shall
be described by its [*] and/or [*].
(iii) For purposes of a listing by Medarex on the Medarex
Exclusive Antigen List, Shared Exclusive Antigen List, or Medarex Non-Exclusive
Antigen List on behalf of Medarex or a Medarex Affiliate, and for purposes of a
listing by Kirin on the Kirin Non-Exclusive Antigen List on behalf of Kirin or a
Kirin Affiliate, and for purposes of each Party's listings in its In-House
Target List (and, in the case of Medarex, the Medarex Internal Project List),
each Antigen shall be
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-33-
described [*], which description shall include the following information to the
extent that such information is [*] to the listing Party at the time of the
listing with respect to such Antigen: the Antigen's [*], other [*], the [*],
equivalent [*] from other [*], and the [*] and/or the [*] therefor; provided,
however, that if there is [*] or equivalent [*] from other [*] with respect to
such Antigen, such Antigen shall be described by its [*] and/or [*].
The Party adding an Antigen to an Antigen List, In-House Target List or Medarex
Internal Project List shall indicate on the list the [*] such Antigen is added
to such list and, where applicable, the [*] that [*] with respect to such
Antigen [*] by such Party or [*] to the other Party, or an Affiliate of either
Party, or a Third Party, as the case may be, whether such Antigen is a [*], and
the [*] in which any [*] to such Antigen are [*].
(b) Antibodies. For purposes of the Antibody Sequence List,
each Antibody shall be identified by reference to the Antibody Amino Acid
Sequence. Medarex shall indicate on such list the date each such Antibody is
added to such list, the date that rights with respect to each such Antibody were
exercised by Medarex or granted to Kirin, an Affiliate of either Party, or a
Third Party, as the case may be, and the respective territory(ies) in which any
rights to such Antibody are held or granted.
(c) Coding System.
(i) [*] than [*] days [*] the Effective Date and during
the remainder of the Term, Medarex shall implement and use the Coding System to
assign codes to all Antigens and Antibodies listed on the Medarex Lists, Shared
Exclusive Antigen List, Medarex In-House Project List and Medarex Internal
Project List and thereafter during the Term shall use the Coding System for
purposes of (i) responding to Antigen Availability Inquiries pursuant to Section
3.3.1(c) hereof, and (ii) responding to Antibody Availability Inquiries pursuant
to Section 3.5.1 hereof.
(ii) From and after the earlier of (A) the date of
implementation of the Coding System by Medarex, or (B) the [*] day of the [*]
day period provided in Section 2.3.1(c)(i) hereof, the code assigned by Medarex
to each Antigen and Antibody hereunder in connection with the Coding System
shall (a) be set forth in any written notice relating to such Antigen or
Antibody required to be provided by Medarex to Kirin pursuant to this Agreement,
and (b) remain on the applicable List in reference to such Antigen or Antibody
and not be changed by Medarex unless Medarex provides to Kirin prior written
notice of such change.
2.3.2 Additions of Antibodies and Antigens to Lists.
(a) Antibodies and Antigens Selected Prior to the Effective
Date.
(i) With respect to each Antibody and each Antigen that
Medarex has selected prior to the Effective Date for Medarex's internal use, or
as to which Medarex has granted a Third Party the right to use any Collaboration
Mice, including, without limitation, the Medarex Mice, Medarex shall include
such Antibody on the Antibody Sequence List and shall include such Antigen on
either the Medarex Restricted Antigen List, Shared Exclusive Antigen List, or
the Medarex Non-Exclusive Antigen List, as appropriate, within [*] business days
after the Effective Date in accordance with Section 2.3.1 hereof; provided,
however, that in the case of any such Antibody, if Medarex has, prior to the
Effective Date, entered into an agreement with a Third Party granting a license
or other rights to one or more such Antibodies with affinity for one or more
Antigens, but such Third Party has not, as of the Effective Date, disclosed to
Medarex the identity of Antibodies with respect to which such Third Party has or
will exercise rights under such agreement, Medarex shall include such Antibodies
on the
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-34-
Antibody Sequence List within [*] business days after any subsequent written
disclosure of any such Antibodies by such Third Party to Medarex; provided,
further, that (1) with respect to any Antigen [*] shall have, at the time of
such listing, (A) [*] with respect to such Antigen, or (B) a [*] with respect to
such Antigen [*]; and (2) with respect to any Antigen listed [*] on the Shared
Exclusive Antigen List [*], or on either the Shared Exclusive Antigen List or
the Medarex Restricted Antigen List [*] shall (A) have, at the time of such
listing, [*] with respect to such Antigen, or (B) [*] with respect to such
Antigen within [*] days after including such Antigen on either the Medarex
Restricted Antigen List or the Shared Exclusive Antigen List, as the case may
be.
(ii) With respect to each Antibody and each Antigen that
Kirin has selected prior to the Effective Date, in reliance on the Agreement for
Essential Terms for Collaboration, for the exercise or grant of a license or
other rights related to any Collaboration Mice, Kirin shall, within [*] business
days after the Effective Date, provide written notice to Medarex identifying
such Antibody(ies) and such Antigen(s) and designate (A) each such Antibody
Kirin has selected in connection with an In-House Project; (B) each such
Antibody Kirin has selected for the grant of a license or other rights to a
Kirin Affiliate or Kirin Partner; (C) each such Antigen Kirin has selected in
connection with an In-House Project; and (D) each such Antigen Kirin has
selected for the grant of a license or other rights to a Kirin Affiliate or
Kirin Partner. Kirin shall indicate with respect to each such Antibody and
Antigen the date on which rights with respect to such Antibody or Antigen were
exercised or granted, as the case may be. Within [*] business days after the
receipt of any such notice(s), Medarex shall include any and all such Antibodies
on the Antibody Sequence List and shall include any and all such Antigens on the
Shared Exclusive Antigen List pursuant to Section 2.3.1 hereof (indicating with
respect to each such Antigen that it is a ROFR Target); provided, however, that
if Kirin exercises a Kirin Non-Exclusive Commercial License with respect to any
such ROFR Target pursuant to Section 4.3.4 hereof, or if Kirin declines to
exercise a Commercial License but elects to retain a Reservation License with
respect to such Antigen pursuant to Section 4.3.3(e)(ii) hereof, it shall be
removed by Medarex from the Shared Exclusive Antigen List and added by Kirin to
the Kirin Non-Exclusive Antigen List, and if Kirin otherwise declines or fails
to exercise a Commercial License with respect to any such ROFR Target pursuant
to Section 4.3.3(e) or 4.3.4 hereof, such Antigen shall be removed by Medarex
from the Shared Exclusive Antigen List.
(b) Antibodies and Antigens Selected On or After the Effective
Date.
(i) With respect to each Antigen (A) that Medarex
selects on or after the Effective Date pursuant to Section 4.1 hereof,
including, without limitation, in connection with a HuMAb Project, or (B) for
which Medarex, on or after the Effective Date, exercises its rights under a
Reservation License pursuant to Section 4.2.2 hereof or a Commercial License
pursuant to Section 4.3 hereof, Medarex shall include such Antigen on either the
Medarex Restricted Antigen List, Shared Exclusive Antigen List, or Medarex
Non-Exclusive Antigen List, as appropriate, within [*] business days after the
date on which Medarex (y) selects such Antigen pursuant to Section 4.1 hereof,
or (z) exercises its rights under a Reservation License or Commercial License
with respect to such Antigen, as the case may be; provided, however, that (1)
with respect to any Antigen [*] shall have, at the time of such listing, (A) [*]
with respect to such Antigen, or (B) a [*] with respect to such Antigen [*]; and
(2) with respect to any Antigen listed [*] on the Shared Exclusive Antigen List
[*], or on either the Shared Exclusive Antigen List or the Medarex Restricted
Antigen List [*] shall (A) have, at the time of such listing, [*] with respect
to such Antigen, or (B) [*] with respect to such Antigen within [*] days after
including such Antigen on either the Medarex Restricted Antigen List or the
Shared Exclusive Antigen List, as the case may be.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-35-
(ii) With respect to each Antigen for which Kirin is
deemed to have exercised its rights under a Reservation License pursuant to
Section 4.2.3 hereof or a Commercial License pursuant to Section 4.3 hereof,
within [*] business days after Kirin's exercise of such rights, Medarex shall
include such Antigen for which Kirin has exercised its rights under an Antigen
Exclusive Commercial License, or an Antigen Semi-Exclusive Commercial License
(or a Reservation License in the case of any Antigen that is a ROFR Target) on
the Shared Exclusive Antigen List. Within [*] business days after the exercise
of its rights under a Reservation License (except in the case of an Antigen that
is a ROFR Target) or Antigen Non-Exclusive Commercial License, Kirin shall
include such Antigen on the Kirin Non-Exclusive Antigen List.
(iii) With respect to each Antibody (A) that Medarex
selects on or after the Effective Date pursuant to Section 4.1 hereof,
including, without limitation, in connection with a HuMAb Project, (B) that
Medarex, on or after the Effective Date, [*] pursuant to Section 4.4.2(a) or
4.4.2(c) hereof, or (C) that Kirin, on or after the Effective Date, [*] pursuant
to Section 4.4.2(b) or 4.4.2(c) hereof, Medarex shall include such Antibody on
the Antibody Sequence List within [*] business days after the date on which (x)
Medarex selects such Antibody pursuant to Section 4.1 hereof, (y) Medarex [*]
such Antibody [*], or (z) Kirin is deemed to have [*] such Antibody [*], as the
case may be; provided, however, that in the case of any such Antibodies, if
Medarex has, [*] entered into an agreement with a Third Party granting a license
or other rights to one or more such Antibodies with affinity for one or more
Antigens, but such Third Party has not, as of the date such Antibody is selected
by such Third Party, disclosed to Medarex the identity of Antibodies with
respect to which such Third Party has or will exercise rights under such
agreement, Medarex shall include such Antibodies on the Antibody Sequence List
within [*] business days after any subsequent written disclosure of any such
Antibodies by such Third Party to Medarex.
2.3.3 Removal of Antibodies and Antigens From Lists.
(a) Antigens That Are the Subject of a License Granted
Pursuant to this Agreement. With respect to any Antigen added to an Antigen List
pursuant to Section 2.3.2 hereof, the Party that maintains such list shall,
within [*] business days after the expiration or termination of the License
relating to such Antigen, remove the reference to such Antigen from any relevant
Antigen List; provided, however, that no such Antigen shall be removed from such
Antigen List in the event that such Antigen is the subject of a Reservation
License and the holder of such License exercises its rights under a Commercial
License with respect to such Antigen pursuant to Section 4.3 hereof, in which
case such Antigen shall be removed from such Antigen List upon the expiration or
termination of the Commercial License; and provided further that no such Antigen
shall be removed from such Antigen List in the event that at least one other
non-exclusive license granted or exercised by a Party relating to such Antigen
remains in effect (or in the event that Medarex has granted or exercised rights
to such Antigen on a non-exclusive basis in connection with a HuMAb Project).
(b) Antibodies That Are [*]. With respect to any
Antibody that is [*] by Medarex or Kirin [*] pursuant to Section 4.4.2(a),
4.4.2(b) or 4.4.2(c) hereof, as applicable, Medarex shall remove such Antibody
from the Antibody Sequence List within [*] business days after (i) any
expiration or earlier termination of the Commercial License to which [*]
relates, or (ii) the [*] with respect to such Antibody, in the case of an
Antibody that is [*] a Party pursuant to Section 4.4.2(c) hereof.
(c) Antigens and Antibodies That Are the Subject of a
HuMAb Project. With respect to any Antibody and/or Antigen that is designated by
Medarex pursuant to Section 4.1 hereof and included in the Antibody Sequence
List or an Antigen List pursuant to Section 2.3.2(a)(i), 2.3.2(b)(i), or
2.3.2(b)(iii) hereof, Medarex shall remove from the Antibody Sequence List and
the
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-36-
relevant Antigen List references to any such Antibody and/or Antigen (i) upon
[*] any [*] Project, [*] Project or [*]; or (ii) upon the expiration or
termination of a license or other rights granted to an Affiliate or Third Party
with respect to such Antibody or Antigen; provided, however, that no such
Antigen shall be removed from an Antigen List in the event that such Antigen,
immediately upon such expiration or termination of such license, shall become
the subject of another license or grant of rights to the holder of the earlier
granted license or rights, or in the event that non-exclusive licenses relating
to such Antigen shall remain in effect. In the event that Medarex, pursuant to
Section 2.3.2(a)(i) or 2.3.2(b)(i) hereof, as applicable, adds an Antigen to the
Shared Exclusive Antigen List in connection with a [*] Project, or to either the
Shared Exclusive Antigen List or the Medarex Restricted Antigen List in
connection with a [*] Project, if [*], Medarex shall remove such Antigen from
the Medarex Restricted Antigen List or the Shared Exclusive Antigen List, as the
case may be, and at its election, add such Antigen to the Medarex Non-Exclusive
Antigen List [*] for itself or the Medarex Affiliate or to grant to such
Third-Party collaborator [*] with respect to such Antigen [*], and unless and
until such Antigen is later determined to be available for the exercise of such
rights in accordance with Article III hereof.
2.3.4 Maintenance of Lists. In the keeping of the Medarex Lists
and the Shared Exclusive Antigen List commencing no later than [*] business days
[*] the Effective Date, Medarex shall utilize a data storage and processing
system that incorporates (a) date stamping functionality, (b) security
functionality, and (c) data integrity functionality (including means to account
for and store the identities of users and the dates on which such users make any
changes to data stored using such system), which system is more fully described
on Exhibit I hereto. All such functionalities with regard to the database shall
meet or exceed the functionalities described on Exhibit I hereto. In any event,
each Party shall exercise no less than reasonable care in maintaining, updating,
handling and storing the Lists, the In-House Target Lists and the Medarex
Internal Project List (as updated from time to time) that such Party is required
to maintain hereunder, and in handling and storing the copies of the Lists, the
In-House Target Lists, and the Medarex Internal Project List provided by one
Party to the other Party pursuant to this Agreement and will take reasonable
steps to safeguard the confidentiality of such lists.
2.3.5 [*].
(a) [*] Agreement and [*]. Promptly following the
Effective Date, the Parties agree to [*] and enter into the [*] Agreement with
such Third Party. Beginning on the effective date of the [*] Agreement, Medarex
shall, [*] pursuant to the terms and conditions of the [*] Agreement. In the
event that the [*] Agreement is terminated for any reason prior to the
termination or expiration of this Agreement, the Parties shall promptly [*],
subject to the terms and conditions of the [*] Agreement.
(b) [*] Under [*] Agreement. Kirin hereby covenants to
Medarex that Kirin will not submit to the [*], pursuant to Section 1.6 of the
[*] Agreement (each a [*]), unless the conditions set forth in this Agreement
with respect to such [*] have been satisfied, including, in the case of a [*],
the conditions of Section 2.6 hereof, and in the case of a [*], Sections
3.3.1(d) and 3.5.2 hereof. Kirin hereby further covenants to Medarex that Kirin
will send to the [*] business day before the expiration of the Additional
Antigen Inquiry Response Period provided for in Section 3.3.1(d)(i) hereof or
the Additional Antibody Inquiry Response Period provided for in Section 3.5.2(a)
hereof, as the case may be [*] business day before the expiration of the
Original Antigen Inquiry Response Period (as defined in Section 3.3.1(d)(i)
hereof) or the Original Antibody Inquiry Response Period (as defined in Section
3.5.2(a) hereof), if the expedited procedure described in the first sentence of
Section 3.3.1(d)(ii) or 3.5.2(b) hereof, as the case may be, has been
triggered), in the event that Medarex has not replied to the relevant Antigen
Availability Inquiry or Antibody Availability Inquiry, as the case may be, prior
to such time.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-37-
2.3.6 [*]
(a) Initial [*] Agreement. Promptly following the
Effective Date, the Parties shall cooperate in good faith to identify a mutually
acceptable Third Party to serve as the [*] and enter into a [*] Agreement with
such Third Party (which Third Party shall satisfy [*], including that the [*] be
an [*]. Such [*] Agreement shall, at a minimum (and in addition to the notice
requirements set forth in Sections 3.3.1(d)(i) and 3.5.2(a) hereof), provide
that:
(i) The [*] shall be engaged by both Parties
[*] of Kirin and Medarex in order to [*] under the circumstances set forth in
Sections 3.3.1(d) and 3.5.2 hereof;
(ii) Medarex shall be obligated, promptly upon
the [*], to [*] with regard to the use of [*], which [*] unless Medarex [*];
provided, however, that Medarex shall not have, and shall expressly disclaim in
the [*] Agreement, any liability relating to such [*] made by the [*] on the
basis of such [*];
(iii) The [*] shall have access to, and make use
of, [*] set forth in Section 3.3.1(d) or Section 3.5.2 hereof;
(iv) The [*] Agreement shall contain
confidentiality provisions customary in the industry prohibiting the [*] from
disclosing the [*] to Kirin or any other Person, except upon being compelled to
do so by a Third Party through legal process;
(v) The [*] Agreement may not be terminated
except upon material breach by the [*] and/or mutual agreement of Kirin and
Medarex.
(b) [*] Form of [*] Agreement. Promptly after the
Parties reach agreement on the initial [*] Agreement pursuant to Section
2.3.6(a) hereof, the Parties shall cooperate in good faith to agree upon an [*]
form of agreement with a [*], for use only in the circumstances contemplated by
Section 2.3.6(c)(ii) hereof. Such [*] form of agreement shall be substantially
in the form of the [*] Agreement entered into by the Parties pursuant to Section
2.3.6(a) hereof, except that such [*] form of agreement shall:
(i) be modified [*];
(ii) in the sole discretion of Medarex, provide
that the [*] in accordance with the terms of such agreement; and
(iii) provide that Medarex is a [*] under such
agreement, with [*] the [*] performance of its obligations thereunder.
From time to time during the Term, in the event that a Party [*] that [*] the
[*] form of agreement initially adopted by the Parties pursuant to the first two
sentences of this Section 2.3.6(b) are [*], such Party shall so inform the other
Party and the Parties shall cooperate in good faith to agree upon [*] to the [*]
form of agreement with a [*], provided that, except with respect to [*] to the
terms relating to [*] by the Parties to the [*], any such [*] form shall be [*]
form of agreement initially adopted by the Parties.
(c) Subsequent [*] Agreements.
(i) Medarex [*]. In the event of termination
of the [*] Agreement, if Kirin desires to engage a [*] at a time when Medarex is
[*], Kirin shall provide to Medarex written
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-38-
notice thereof, which notice shall be delivered by internationally recognized
courier to the recipients identified in Section 18.3.2 hereof. In such event,
the [*] shall be [*], provided that the [*] shall satisfy the [*]. Promptly
following the [*] Agreement with such Person in a form that is [*] initially
executed by the Parties pursuant to Section 2.3.6(a) hereof, provided that any
changes to the form initially executed by the Parties shall [*].
(ii) Medarex [*]. In the event of termination
of the [*] Agreement, if Kirin desires to engage a [*] at a time when Medarex is
[*], Kirin shall select a [*] that satisfies the [*] and provide to Medarex
written notice that Kirin desires for Medarex to enter into a [*] Agreement with
such Third Party, which notice shall be delivered by internationally recognized
courier to the recipients identified in Section 18.3.2 hereof. As part of such
notice, Kirin shall provide to Medarex for signature a [*] Agreement signed by
Kirin and such [*], which agreement shall be in the form of [*] Agreement
executed by Medarex and Kirin pursuant to Section 2.3.6(a) hereof. In the event
that Medarex does not sign and return the [*] Agreement to Kirin within [*] days
of receiving it from Kirin, Kirin shall provide to Medarex written notice of
Medarex's failure to respond to Kirin's request that Medarex sign the [*]
Agreement, which notice shall be delivered by internationally recognized courier
to the recipients identified in Section 18.3.2 hereof and shall contain the
following legend, printed in fourteen (14) point or larger font, capital letters
and boldface type immediately below the recipients names and addresses:
"IMPORTANT. SECOND NOTICE. REFER TO 2.3.6 OF THE MEDAREX-KIRIN AGREEMENT FOR
CONSEQUENCES OF FAILURE TO RESPOND". As of the date on which such notice is
received by Medarex, Medarex shall have an [*] business days to sign and return
to Kirin the [*] Agreement. [*].
(d) Kirin Not Permitted to Access [*]. It is
understood and agreed that nothing contained in this Agreement or the [*]
Agreement shall permit Kirin to obtain access to the [*] or the information
contained therein.
2.4 Disclosure of Certain Antigen Lists. Within [*] business days
[*] the Effective Date, Medarex shall provide to Kirin a true, correct and
complete copy of the Shared Exclusive Antigen List, and Kirin shall provide to
Medarex a true, correct and complete copy of the Kirin Non-Exclusive Antigen
List. In addition, from time to time during the Term of this Agreement, but [*]
in which any amendment or other change is made thereto, each Party shall provide
the other Party with true, correct and complete updated copies of such List(s),
if any. If requested by Medarex, Kirin shall provide a true, correct and
complete updated copy of the Kirin Non-Exclusive Antigen List as frequently as
Medarex may reasonably require, but [*] business days, as necessary to enable
[*]. It is understood and agreed that Medarex shall not be required to provide
or otherwise disclose to Kirin the Medarex Restricted Antigen List, Medarex
Non-Exclusive Antigen List, or Antibody Sequence List; provided, however, that
Medarex shall maintain such lists in accordance with Section 2.3 hereof and
Kirin shall be permitted to [*].
2.5 Confidentiality of Antigen and Antibody Disclosures. The
disclosure of any Antigen List pursuant to Section 2.4 hereof, any disclosure by
a Party to the other Party of the identity of an Antibody or an Antigen in
connection with this Agreement, and any information provided by one Party to the
other Party in response to any availability inquiries made pursuant to Article
III hereof shall be subject to the terms and conditions of Article XIII hereof.
Each Party represents, warrants, and covenants to the other Party that it will
[*] provided by one Party to the other Party in response to Antigen and
Antibody-related inquires, and will [*] of such information.
2.6 Records Retention; [*].
2.6.1 Record Retention. The Party with an obligation to
establish and maintain any List(s) pursuant to Section 2.1.1 or 2.1.2 hereof and
to make and/or respond to Antigen or Antibody
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-39-
availability inquiries pursuant to Sections 3.2.1, 3.3.1, 3.4 or 3.5 hereof,
shall during the Term of this Agreement and for a period of [*] years after its
expiration or earlier termination keep records of Antibodies and Antigens added
and removed from such List(s) and availability inquiries received and
availability responses provided, and the dates of the foregoing, in sufficient
detail to confirm the timeliness and accuracy of such entries, deletions and
availability responses.
2.6.2 [*]. Upon the written request of the other Party and not
more often than once every [*] months, the Party maintaining such List(s) and/or
required to respond to availability inquiries by the other Party shall [*], to
all of the records of the Party that relate to such List(s) and/or such
inquiries as may be [*] thereof (including, as necessary, any information from
any database or Lists maintained by such Party and other information maintained
by such Party pursuant to Section 2.6.1 hereof).
2.6.3 [*] Records. If the [*] that the [*] Party [*] or
otherwise [*] to [*] an [*] or [*] on such [*] an [*] or [*] from such [*], or
[*] and/or [*] (any of the foregoing, a [*]), the [*] shall [*] that such a [*]
and shall [*]. In the event that (a) the [*] (including the [*] thereof) to the
[*] of the [*] (as defined below), and the [*] (including the [*] thereof) [*],
or (b) if such [*] is not capable of cure and adversely affects the [*] Party,
then in either event the [*] to enforce its rights hereunder. For purposes of
this Section 2.6.3, [*] shall mean [*] days from the date on which the [*] Party
receives from the [*], or in the event that the [*] (including the [*] thereof)
cannot, despite [*], be [*] within such period, [*] days from the date on which
the [*] Party receives from the [*] (provided that such [*] day period shall
apply only in the event and for so long as the [*] Party continues to [*]). With
respect to any [*] pursuant to this Section 2.6.3, (i) the Parties shall [*] and
failing [*] within a [*], either Party may [*]; and (ii) upon the [*] Party's
reasonable request, the [*] Party shall take reasonable further actions to [*]
from arising in the future.
2.6.4 [*] Except as provided in this Section 2.6.4, the [*]
Party shall treat all [*] in connection with such [*] as [*] of the [*] Party in
accordance with [*] hereof. With respect to any [*] to the [*] Party by the [*]
that [*], the [*] Party shall continue to [*] such [*] in accordance with [*]
hereof and in addition shall [*] to [*] and [*] such information in the [*] and
shall provide [*] such information [*] to its [*] and a [*] of [*] and only to
the extent that such persons have [*] such information to [*] in [*] such [*]
Party's rights hereunder. Notwithstanding the fact that information [*] Party
pursuant to this Section 2.6 is [*], the [*] Party shall have a right to the
extent [*] to [*] hereunder to [*] to it by the [*] arising under this Agreement
concerning a [*] but shall in each instance [*] to [*] of such information,
including by [*] to [*] and/or other [*] such information in any such [*]. The
[*] Party shall cause the [*] to enter into a [*] with, and in a form reasonably
acceptable to, the [*] Party and require the [*] to the [*] in connection with
the [*].
ARTICLE III
ANTIBODY AND ANTIGEN AVAILABILITY
3.1 Principles of Antibody and Antigen Availability. The Parties agree to
comply with the principles of Antibody and Antigen availability set forth in
this Article III in connection with responding to availability inquiries made by
the other Party, and making any grant to Affiliates and/or Third Parties of, or
exercising, rights in, to or under the Technology, KM Patent Rights and/or KM
Know How and relating to any Collaboration Mice for use in the research,
development and/or commercialization of Collaboration Antibodies and
Collaboration Products, as contemplated in this Agreement. The Parties further
agree that any and all investigations and conclusions made pursuant to this
Article III with respect to the availability or unavailability of such Antigen
and/or Antibody in response to an inquiry by the other Party, or for the grant
to Affiliates and/or Third Parties, or the exercise by a Party, of rights shall
(i) be
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-40-
made [*], as [*], and (ii) be [*] basis as that concept is more fully described
in the following Sections. The Parties acknowledge and agree that this Section
3.1 is meant to describe in general terms the principles for determining
availability and that nothing in this Section 3.1 is intended to alter or
override more specific provisions contained in this Agreement.
3.2 Medarex Antigen [*].
3.2.1 Review Procedure. During the Term of this Agreement, in the
event that Medarex desires to [*] of an Antigen [*], or [*] with which [*] in
connection with [*] pursuant to Section 4.1 hereof, Medarex shall review (a)
each of the Antigen Lists maintained by Medarex pursuant to Section 2.1.1
hereof, and, if Medarex desires to [*] of an Antigen on an [*] basis, the latest
version of the [*] as provided to [*] pursuant to Section 2.4 hereof; and (b)
any and all notices, inquiries and other [*] an Antigen that have been [*] as of
the [*] at which [*] pursuant to clause (a) hereof but which Antigen(s) has [*]
in accordance with Article II hereof. Prior to [*] with respect to such Antigen
on an [*] basis, Medarex shall [*] that [*], and [*] within [*] business days
after the receipt of such request, to enable Medarex to confirm whether there
have been any [*] since a [*], and, if so, [*] to such list.
3.2.2 Available Antigens.
(a) Unlimited Availability. An Antigen shall be available to
Medarex for (i) the grant or exercise of rights pursuant to Section 4.1 hereof
on an exclusive or non-exclusive basis, (ii) the exercise of its rights under a
Reservation License pursuant to Section 4.2 hereof, or (iii) the exercise of its
rights under a Commercial License pursuant to Section 4.3 hereof, if [*] with
respect to an Antigen pursuant to Section 3.2.1 hereof or, if [*] with respect
to such Antigen, (y) rights with respect to such Antigen [*], as confirmed by
Medarex's review of the information on the then-current [*] and on the
then-current [*], and (z) Medarex [*] indicating that [*] desires to exercise
its rights under a License with respect to such Antigen.
(b) Limited Availability. An Antigen shall be available to
Medarex for the exercise of its rights (i) pursuant to Section 4.1 hereof [*]
(e.g., on a [*], or subject to any [*] on a [*], (ii) under a [*] pursuant to
Section [*] hereof, (iii) under an [*] pursuant to Section [*] hereof, or (iv)
under an [*] pursuant to Section [*] hereof, if as of the [*] at which [*]
pursuant to Section 3.2.1 hereof or, if the [*]y, as of the [*] at which [*]
with respect to such Antigen, (y) rights with respect to such Antigen [*], as
confirmed by information on the then-current [*] and on the then-current [*],
and (z) Medarex [*] indicating that [*] desires to exercise its rights under a
License with respect to such Antigen [*].
3.2.3 Unavailable Antigens. An Antigen shall be unavailable to
Medarex for the grant or exercise of rights pursuant to Article IV hereof if
[*], or, in the case of an [*], (a) rights with respect to such Antigen [*], as
confirmed by information on the then-current [*], or (b) Medarex [*] indicating
that [*] desires to exercise its rights under a License with respect to such
Antigen [*].
3.2.4 Antigens Subject to ROFRs. Notwithstanding the foregoing, if
as of the [*] of an availability inquiry by Medarex with respect to an Antigen
pursuant to Section 3.2.1 hereof, such Antigen is a ROFR Target for which
Refusal Rights are held by [*], as confirmed by Medarex's review of the
information on the then-current [*] maintained by Medarex and Kirin, and the
other information that Medarex is required to review pursuant to Section 3.2.1
hereof, then such Antigen shall be unavailable to Medarex for the [*] pursuant
to Article [*] hereof [*] hereof, as applicable, are satisfied with respect to
such Antigen.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-41-
3.2.5 Medarex Right to Make [*] Inquiries. Subject to Section 3.7
hereof, Medarex shall be permitted during the Term of the Agreement to make [*]
inquiries with respect to the availability of Antigens pursuant to Section 3.2.1
hereof, [*] to [*] or to otherwise [*] with respect to such Antigen(s) pursuant
to Section [*] hereof.
3.3 Kirin Antigen Inquiries.
3.3.1 Inquiry Procedures.
(a) Antigen Availability Inquires by Kirin. During the Term
of this Agreement, in the event that Kirin desires, on behalf of itself, or any
of its Affiliates, Partners or In-House Collaborators, to ascertain the
availability of an Antigen, Kirin shall review the Kirin [*] List and shall make
an Antigen Availability Inquiry to Medarex indicating in such inquiry whether it
seeks to determine the availability of such Antigen on an exclusive,
semi-exclusive, or non-exclusive basis. For the avoidance of doubt, as part of a
[*] Kirin may inquire as to whether the Antigen [*].
(b) Review of Information by Medarex. In response to an
Antigen Availability Inquiry, Medarex shall investigate whether and to what
extent such Antigen is available by reviewing (i) each of the then-current [*]
maintained by Medarex pursuant to Section 2.1.1 hereof and the Kirin [*] List,
and (ii) any and all written notices, written inquiries and other written
requests for rights with respect to an Antigen that have been received by
Medarex from a Medarex Affiliate, Kirin or a Third Party [*] by Medarex of the
subject Antigen Availability Inquiry from Kirin pursuant to Section 3.3.1(a)
hereof but which Antigen(s) has not yet been added to the Lists maintained by
Medarex in accordance with Article II hereof.
(c) Medare x Response to Kirin. [*] after making an
availability investigation in response to an Antigen Availability Inquiry from
Kirin, but in no event [*] business days following receipt of such inquiry,
Medarex shall inform Kirin whether the Antigen is available on the basis as to
which Kirin has inquired, as determined in accordance with Sections 3.3.2, 3.3.3
and 3.3.4 hereof, and with respect to each Antigen that is unavailable to Kirin
on the basis as to which Kirin has inquired, Medarex shall also disclose to
Kirin the code assigned to such Antigen by Medarex in accordance with the Coding
System at the time Medarex informs Kirin of the unavailability of such Antigen.
In the event that Kirin desires to exercise an Antigen Exclusive Commercial
License with respect to a particular Antigen and the Antigen is not available
for the exercise of such a license, or in the event that Kirin desires to
exercise an Antigen Semi-Exclusive Commercial License or an Antigen
Non-Exclusive Commercial License with respect to a particular Antigen and the
Antigen is not available for the exercise of either such license, then [*] shall
[*] provide to [*] evidencing the [*] or [*] of [*] to such Antigen, subject to
[*] to [*] the [*], information other than the information required to confirm
such [*] or [*] of [*], and any other [*] of any such [*]. In the event that
Kirin has inquired about the availability of an Antigen for the exercise of an
Antigen Exclusive Commercial License or an Antigen Semi-Exclusive Commercial
License, and such Antigen is not available for an Antigen Exclusive Commercial
License but is available for an Antigen Semi-Exclusive Commercial License, [*]
shall [*] as to whether [*] than [*] has [*] with [*] to an [*] (but shall not
be required to [*] the [*] of [*] which have such [*]). In the event that Kirin
has inquired about the availability of an Antigen for the exercise of a License
and such Antigen is not available because it is a ROFR Target, [*] shall [*],
and except to the extent [*] to the [*] which holds the [*], as determined in
[*] shall [*] of the steps required to [*] and the [*] such [*] has to [*].
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-42-
(d) Consequence of Medarex's Failure to Respond to Antigen
Availability Inquiry.
(i) In the event that Medarex does not respond to
an Antigen Availability Inquiry within the applicable [*] business day period
(with respect to each Antigen Availability Inquiry, the "Original Antigen
Inquiry Response Period"), and Kirin continues to desire a response to such
inquiry, Kirin shall provide to Medarex written notice of Medarex's failure to
respond, which notice shall be delivered by an internationally recognized
courier to the recipients identified in Section 18.3.2 hereof and shall contain
the following legend, printed in fourteen (14) point or larger font, capital
letters and boldface type immediately below the recipients' names and addresses:
"IMPORTANT. SECOND NOTICE. REFER TO SECTION 3.3.1(d) OF THE MEDAREX-KIRIN
AGREEMENT FOR CONSEQUENCES OF FAILURE TO RESPOND." Subject to Section
3.3.1(d)(ii) hereof, as of the date on which such notice is received by Medarex,
Medarex shall have an [*] business day period to respond to the Antigen
Availability Inquiry (with respect to each Antigen Availability Inquiry, the
"Additional Antigen Inquiry Response Period"). In the event that [*] within the
[*], Kirin shall have [*] the [*] pursuant to the terms of the [*]. Until such
time as [*] shall deliver to Kirin written notice of [*] to Kirin for the
exercise of the rights about which Kirin has inquired in the applicable Antigen
Availability Inquiry in accordance with the terms of the [*] Agreement, the
Antigen that is the subject of the Antigen Availability Inquiry shall be
unavailable to Kirin for the exercise of such rights pursuant to Article IV
hereof. The [*] Agreement shall require that, in the event that the [*], the [*]
shall provide notice of that [*] to Kirin and shall provide a copy of such [*]
to Medarex. For purposes of this Agreement, a [*], provided that such [*] is
made in accordance with the terms and conditions of this Section 3.3.1(d) and
the [*] Agreement.
(ii) In the event Medarex does not respond [*]
Antigen Availability Inquiries, in each case during the Original Antigen Inquiry
Response Period and the Additional Antigen Inquiry Response Period, and
Medarex's initial receipt of the first and the last of such Antigen Availability
Inquiries is separated by at [*], Kirin shall have the right with respect to
each subsequent Antigen Availability Inquiry for which Medarex fails to respond
during the Original Antigen Inquiry Response Period [*] pursuant to Section
3.3.1(d)(i) hereof and the terms of the [*] Agreement (without waiting for the
Additional Antigen Inquiry Response Period to lapse with respect to such
inquiry). Notwithstanding the foregoing, in the event that, in response to
subsequent Antigen Availability Inquiries from Kirin, Medarex responds to at
least [*] Antigen Availability Inquiries, in each case during the Original
Antigen Inquiry Response Period, and Medarex's initial receipt of the first and
the last of such Antigen Availability Inquiries is separated by at [*] weeks,
the provisions of the preceding sentence shall cease to apply until and unless
Medarex again does not respond to [*] Antigen Availability Inquiries, in each
case during the Original Antigen Inquiry Response Period and the Additional
Antigen Inquiry Response Period, and Medarex's initial receipt of the first and
the last of such Antigen Availability Inquiries is separated by [*] weeks, in
which case the preceding sentence shall again apply.
(iii) In the event that Medarex does not respond to
an Antigen Availability Inquiry prior to the expiration of the Additional
Antigen Inquiry Response Period with respect to such inquiry (or prior to the
expiration of the Original Antigen Inquiry Response Period if the expedited
procedure described in the first sentence of Section 3.3.1(d)(ii) hereof has
been triggered), and as of the [*] of such period there is not a [*] Agreement
in effect, [*].
3.3.2 Available Antigens.
(a) Unlimited Availability. An Antigen is available to Kirin for
the exercise of its rights under (i) a Reservation License pursuant to Section
4.2 hereof, or (ii) any Commercial License
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-43-
pursuant to Section 4.3 hereof, if [*] with respect to such Antigen pursuant to
Section 3.3.1(a) hereof, (y) rights with respect to such Antigen [*], as
confirmed by the information on the then-current [*] maintained [*] and on the
then-current [*], and (z) Medarex [*] from any [*] or [*] with which [*] has [*]
an [*] pursuant to which such [*] or [*] has [*] against which to [*], a [*] for
a [*] or other [*] within the [*] of such [*] with respect to [*]. For the
avoidance of doubt, in order for the [*] referred to in [*] to render an Antigen
[*], under the terms of the [*], such [*] thereto must [*] the [*] or [*] with
respect to such Antigen within [*] business days from the date on which Medarex
notifies [*]; provided, however, that with respect to [*], the [*] shall have
[*] as may be permitted under [*] to obtain or exercise such rights.
(b) Limited Availability. An Antigen shall be available
to Kirin for the exercise of its rights under (i) a Reservation License pursuant
to Section 4.2 hereof, (ii) an Antigen Semi-Exclusive Commercial License
pursuant to Section 4.3 hereof, or (iii) an Antigen Non-Exclusive Commercial
License pursuant to Section 4.3 hereof, if [*] with respect to such Antigen
pursuant to Section 3.3.1(a) hereof, (y) rights with respect to such Antigen
[*], as confirmed by information on the then-current [*], and (z) Medarex [*]
from any [*] or [*] with which [*] has [*] an [*] pursuant to which such [*] or
[*] has [*] against which to [*], a [*] for a [*] or other [*] within the [*] of
such [*] with respect to such [*] on an [*] basis. For the avoidance of doubt,
in order for the [*] to render an Antigen unavailable to any extent, under the
terms of the [*], such [*] thereto must [*] of the [*] or [*] with respect to
such Antigen within [*] business days from the date on which Medarex notifies
[*]; provided, however, that with respect to [*], the [*] shall have [*] as may
be permitted under [*] to obtain or exercise such rights.
3.3.3 Unavailable Antigens. An Antigen shall be unavailable
to Kirin for the grant or exercise of rights pursuant to Article IV hereof if
[*] with respect to such Antigen pursuant to Section 3.3.1(a) hereof, (a) rights
with respect to such Antigen [*], as confirmed by information on the
then-current [*], or (b) Medarex [*]. For the avoidance of doubt, in order for
[*] to render an Antigen unavailable to any extent, under the terms of the [*],
such [*] with respect to such Antigen within [*] business days from the date on
which Medarex notifies [*]; provided, however, that with respect to [*], the [*]
with which Medarex has entered into [*] shall have [*] as may be permitted under
[*] to obtain or exercise such rights.
3.3.4 Antigens Subject to ROFRs. Notwithstanding the
foregoing, if [*] with respect to an Antigen pursuant to Section 3.3.1(a)
hereof, such Antigen has become a ROFR Target for which Refusal Rights are held
by [*], as confirmed by Medarex's review of the information on the [*]
maintained by Medarex and Kirin, and the other information that Medarex is
required to investigate pursuant to Section 3.3.1(b) hereof, then such Antigen
shall be unavailable to Kirin for the exercise of rights under Article IV hereof
unless and until the requirements of Section 4.3.4(d) or (e) hereof, as
applicable, are satisfied with respect to such Antigen.
3.3.5 Kirin Right to Make [*] Inquiries. Subject to Section
3.7 hereof, Kirin shall be permitted during the Term of the Agreement to make
[*] Antigen Availability Inquiries pursuant to Section 3.3.1(a) hereof, [*] to
obtain a Reservation License or a Commercial License.
3.4 Medarex Antibody Inquiries.
3.4.1 Review Procedures. During the Term of this Agreement,
in the event that Medarex desires to ascertain the availability of an Antibody
on behalf of itself, any of its Affiliates, Partners, In-House Collaborators or
other Third Parties with which it enters into a Project Agreement in connection
with one or more HuMAb Projects pursuant to Section 4.1 hereof, Medarex shall
review
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-44-
(i) the [*] (which, in the case of any [*], shall be reviewed to identify
whether [*] with respect to the Antigen against which such Antibody was raised),
and (ii) any and all written notices, written inquiries and other written
requests for rights with respect to an Antibody and/or Antigen-exclusive or
Antigen-semi-exclusive rights with respect to an [*] pursuant to this Section
3.4.1 but which Antibody(ies) and/or Antigen(s) have not yet been added to the
[*] in accordance with Article II hereof.
3.4.2 Available Antibodies. An Antibody shall be available to
Medarex for the grant or exercise of rights pursuant to Sections 4.1 and 4.4
hereof if, [*] pursuant to Section 3.4.1 hereof or, if [*] with respect to such
Antibody, (i) such Antibody [*] List, and the Antigen against which such
Antibody was raised and for which such Antibody has affinity [*]; and (ii)
Medarex [*] pursuant to Section 4.4.2(b) or 4.4.2(c) hereof indicating that [*]
desires to [*] such Antibody [*] or [*] pursuant to Section 4.3 hereof
indicating that [*] desires to exercise an Antigen Exclusive Commercial License
or an Antigen Semi-Exclusive Commercial License with respect to the Antigen
against which such Antibody was raised.
3.4.3 Unavailable Antibodies. An Antibody shall be
unavailable to Medarex for the grant or exercise of rights pursuant to Section
4.1 or 4.4 hereof, if, [*] pursuant to Section 3.4.1 hereof or, if [*], (i) such
Antibody has been listed on the then-current [*], or the Antigen against which
such Antibody was raised, and for which such Antibody has affinity [*]; or (ii)
Medarex has received from Kirin [*] to Section 4.4.2(b) or 4.4.2(c) hereof
indicating that [*] desires to [*] such Antibody [*] or a [*] pursuant to
Section 4.3 hereof indicating that [*] desires to exercise an Antigen Exclusive
Commercial License or an Antigen Semi-Exclusive Commercial License with respect
to the Antigen against which the Antibody was raised and for which such Antibody
has affinity. Such Antibody shall continue to be unavailable to Medarex unless
and until such Antibody is later determined to be available in connection with a
subsequent Antibody review. Subject to Section 3.7 hereof, there shall be [*]
Antibody inquiries that Medarex can make without [*] pursuant to Section 4.4
hereof or otherwise [*] with respect to such Antibody pursuant to Section 4.1
hereof.
3.5 Kirin Antibody Inquiries.
3.5.1 Inquiry Procedures. During the Term of this Agreement,
in the event that Kirin desires to ascertain the availability of an Antibody on
behalf of itself, any of its Affiliates, Partners, or In-House Collaborators,
Kirin shall provide an Antibody Availability Inquiry to Medarex. With respect to
an Antibody Availability Inquiry for an Antibody raised against and with
affinity for a Kirin Reservation Target, Kirin Exclusive Commercial Target,
Kirin Non-Exclusive Commercial Target, or Kirin Semi-Exclusive Commercial
Target, within [*] business days following receipt of such inquiry from Kirin,
Medarex shall (i) review the then current [*] and any and all [*] and other [*]
for [*] that have been [*] from a [*] or [*] as of the [*] of [*] from [*] of
such [*] pursuant to this Section 3.5.1 but which Antibody(ies) has not yet been
added to the [*] List in accordance with Article II hereof, and (ii) inform
Kirin whether the Antibody is available. With respect to an Antibody
Availability Inquiry for an Antibody raised against and with affinity for an
Antigen, other than an Antigen that is a Kirin Reservation Target or a Kirin
Commercial Target, within [*] business days following receipt of such inquiry
from Kirin, Medarex shall (i) review the then-current [*] (which, in the case of
any [*], shall be [*] whether any [*] (other than the [*] the [*] is [*]) has
[*] or [*] or [*] with respect to [*], and any and all [*] and other [*] for [*]
with respect to an [*] and/or [*] or [*] with respect to an [*] that have been
[*] from a [*] or [*] as of the [*] of [*] from [*] of such [*] pursuant to this
Section 3.5.1 but which Antibody(ies) and/or Antigen(s) have not yet been added
to the [*] or [*] in accordance with Article II hereof, and (ii) inform Kirin
whether the Antibody is available. With respect to each Antibody that is
unavailable to Kirin as determined in accordance with Section 3.5.4 hereof,
Medarex shall also disclose to Kirin the code assigned by Medarex to such
Antibody (and the code assigned to the Antigen against which such
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-45-
Antibody was raised) in accordance with the Coding System at the time Medarex
informs Kirin of the unavailability of such Antibody. In the event that such
Antibody is not available, [*] shall [*] to [*] evidencing the [*] or [*] of [*]
to such Antibody, subject to [*] to [*] the [*] of any [*] or [*], information
other than the information [*] such [*] or [*] of [*], and any other [*] of any
[*] or [*].
3.5.2 Consequence of Medarex's Failure to Respond to Antibody
Availability Inquiry.
(a) In the event that Medarex does not respond to an
Antibody Availability Inquiry within the applicable [*] business day period (as
provided in Section 3.5 hereof) (with respect to each Antibody Availability
Inquiry, the "Original Antibody Inquiry Response Period"), and Kirin continues
to desire a response to such inquiry, Kirin shall provide to Medarex written
notice of Medarex's failure to respond, which notice shall be delivered by an
internationally recognized courier to the recipient identified in Section 18.3.2
hereof and shall contain the following legend, printed in fourteen (14) point or
larger font, capital letters and boldface type immediately below the recipients'
names and addresses: "IMPORTANT. SECOND NOTICE. REFER TO SECTION 3.5.2 OF THE
MEDAREX-KIRIN AGREEMENT FOR CONSEQUENCES OF FAILURE TO RESPOND". Subject to
Section 3.5.2(b) hereof, as of the date on which such notice is received by
Medarex, Medarex shall have an [*] business day period to respond to the
Antibody Availability Inquiry, in any case where the Original Antibody Inquiry
Response Period was [*] business days, and an [*] business day period to respond
to the Antibody Availability Inquiry, in any case where the Original Antibody
Inquiry Response Period was [*] business days (with respect to each Antibody
Availability Inquiry, the "Additional Antibody Inquiry Response Period"). In the
event that [*] within [*] shall have the [*] pursuant to the [*] of the [*]
Until such time as the [*] shall deliver to Kirin written notice of [*] in
accordance with the terms of the [*] Agreement, the Antibody that is the subject
of the Antibody Availability Inquiry shall be unavailable to Kirin for the
exercise of such rights pursuant to Article IV hereof. The [*] Agreement shall
require that, in the event that the [*], the [*] shall provide notice of that
[*] to Kirin and shall provide a copy of such [*] to Medarex. For purposes of
this Agreement, a [*], provided that such [*] is made in accordance with the
terms and conditions of this Section 3.5.2 and the [*] Agreement.
(b) In the event that Medarex does not respond to [*]
Antibody Availability Inquiries, in each case during the Original Antibody
Inquiry Response Period and the Additional Antibody Inquiry Response Period, and
Medarex's initial receipt of the first and the last of such Antibody
Availability Inquiries is [*], Kirin shall have the right with respect to each
subsequent Antibody Availability Inquiry for which Medarex fails to respond
during the Original Antibody Inquiry Response Period to have [*] pursuant to
Section 3.5.2(a) hereof and the terms of the [*] Agreement (without waiting for
the Additional Antibody Inquiry Response Period to lapse with respect to such
inquiry). Notwithstanding the foregoing, in the event that, in response to
subsequent Antibody Availability Inquiries from Kirin, Medarex responds to [*]
Antibody Availability Inquiries, in each case during the Original Antibody
Inquiry Response Period, and Medarex's initial receipt of the first and last of
such Antibody Availability Inquiries is [*], the provisions of the preceding
sentence shall cease to apply until and unless Medarex again does not respond to
[*] Antibody Availability Inquiries, in each case during the Original Antibody
Inquiry Response Period and the Additional Antibody Inquiry Response Period, and
Medarex's initial receipt of the first and last of such Antibody Availability
Inquiries is [*] weeks, in which case the preceding sentence shall again apply.
(c) In the event that Medarex does not respond to an
Antibody Availability Inquiry prior to the expiration of the Additional Antibody
Inquiry Response Period with respect to such inquiry (or prior to the expiration
of the Original Antibody Inquiry Response Period if the expedited
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-46-
procedure described in the first sentence of Section 3.5.2(b) hereof has been
triggered), and as of the [*] of such period there is not a [*] Agreement in
effect, [*].
3.5.3 Available Antibodies. An Antibody shall be available to
Kirin for the grant or exercise of rights pursuant to Section 4.4 hereof if [*]
(i) such Antibody [*], and the Antigen against which such Antibody was raised
[*], and (ii) [*]. For the avoidance of doubt, in order for the [*] to render an
Antibody unavailable, under the terms of the [*], such [*] with respect to such
Antibody [*] business days from the date on which Medarex notifies [*];
provided, however, that with respect to [*], the [*] shall have [*] as may be
permitted under [*] to obtain or exercise such rights.
3.5.4 Unavailable Antibodies. An Antibody shall be unavailable
to Kirin for the exercise of rights pursuant to Section 4.4 hereof if [*] (i)
such Antibody [*], or the Antigen against which such Antibody was raised [*], or
(ii) Medarex [*]. Such Antibody shall continue to remain unavailable to Kirin
unless and until such Antibody is later determined to be available in connection
with a subsequent Antibody Availability Inquiry. Subject to Section 3.7 hereof,
there shall be [*] Antibody Availability Inquiries that Kirin can make to
Medarex, [*] to include any such Antibody [*] pursuant to Section 4.4 hereof.
For the avoidance of doubt, in order for [*] to render an Antibody unavailable,
under the terms of the [*], such [*] with respect to such Antibody within [*]
business days from the date on which Medarex notifies [*]; provided, however,
that with respect to [*], the [*] with which Medarex has entered into [*] shall
have [*] as may be permitted under [*] to obtain or exercise such rights.
3.6 Notices, Inquiries and Other Requests Relating to Antibodies and
Antigens. In order to ensure accurate determinations by Medarex with respect to
the priority of receipt of any notices, inquiries, and other requests from
Kirin, Medarex Affiliates and Third Parties for rights with respect to Antigens
and Antibodies, Medarex shall [*] in documenting and maintaining such notices,
inquiries and other requests, [*]. For purposes of this Article III and Article
IV hereof, notices, inquiries and other requests for rights relating to
Antibodies and Antigens that are provided to Medarex by Kirin, any Medarex
Affiliates or Third Parties, shall be deemed to be "received" by Medarex on the
date and time at which the notice, inquiry or request is actually received in
writing by Medarex (which in the case of notices, inquiries and requests made by
Kirin shall comply with Section 18.3.3 hereof), except to the extent that the
terms of any applicable agreement entered into between Medarex and a Third Party
prior to the Effective Date provide that the notice, inquiry, or request shall
be deemed made by such Third Party and/or deemed received by Medarex on a
different date (which date shall not be more than [*] days prior to the date of
actual receipt of such notice), in which case the date for determining priority
with respect to Antigen or Antibody availability shall be determined in
accordance with the terms of such agreement. Unless otherwise expressly
indicated, "written", when referring to any notice, inquiry or request under
this Article III, include writings on paper, delivered by mail, courier,
facsimile or otherwise, as well as electronic transmissions (e.g., via email).
3.7 Covenants Relating to Lists and Disclosures. Medarex covenants that
it will not use the information contained in the Lists or the information
disclosed by Kirin to Medarex in accordance with Sections 6.5.1 or 6.5.2 hereof,
or otherwise make availability inquiries pursuant to Section 3.2.1 or 3.4,1
hereof, for the purpose of identifying Antigens or Antibodies for Exploitation
by Medarex or any of its Affiliates, Partners, In-House Collaborators, or Third
Parties with which Medarex enters into a Project Agreement in connection with
one or more HuMAb Projects pursuant to Section 4.1 hereof. Kirin covenants that
it will not use the information contained in the Lists, the information
disclosed by Medarex to Kirin in response to Antigen Availability Inquiries or
Antibody Availability Inquiries, or the information disclosed by Medarex to
Kirin in accordance with Sections 6.5.1 or 6.5.2 hereof, or otherwise make
inquiries to Medarex pursuant to Section 3.3.1(a) or 3.5.1 hereof, for the
purpose of identifying Antigens or Antibodies for Exploitation by Kirin or any
of its Affiliates, Partners, or In-House
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-47-
Collaborators. For the avoidance of doubt, once a Party, without reference to
the Lists or the other Antigen or Antibody-related information disclosed by a
Party to the other Party pursuant to this Article III hereof, has identified an
Antigen or an Antibody as a possible candidate for Exploitation, it may use the
information contained in the Lists for the purpose of determining whether it is
permitted, pursuant to the terms of this Agreement, to proceed with the
Exploitation of such Antibody or Antigen, as the case may be.
3.8 Purchase of Rights. Subject to all the other terms and conditions of
this Agreement, including restrictions on sublicensing contained in Article VIII
hereof, nothing set forth in Sections 3.2.3, 3.3.3, 3.4.3 or 3.5.4 hereof is
intended to limit either Party's right to independently purchase or in-license
rights from any Person.
ARTICLE IV
ANTIGEN AND ANTIBODY SELECTION FOR EXERCISE OF LICENSE RIGHTS
4.1 Projects Involving Use of Medarex Mice and/or Medarex Technology. In
the event and to the extent that an Antigen is available pursuant to Section
3.2.2(a) or 3.2.2(b) hereof, or that an Antibody is available pursuant to
Section 3.4.2 hereof, in addition to Medarex's rights under Sections 4.2, 4.3,
and 4.4 hereof, during the Term of this Agreement Medarex retains and shall have
the right to use the Medarex Technology and Medarex Mice, and/or to grant to one
or more Medarex Affiliates or Third Parties rights to use the Medarex Technology
and/or Medarex Mice, in connection with the research, development and/or
commercialization of any such Antigen or any such Antibody selected by Medarex
pursuant to this Section 4.1 and/or Antibody Products containing any such
Antibody (and Antibody Material related thereto), including, without limitation,
in connection with a HuMAb Project; provided, however, that Medarex shall add
such Antigen or Antibody on either the Medarex Restricted Antigen List, Shared
Exclusive Antigen List, Medarex Non-Exclusive Antigen List or Antibody Sequence
List, as appropriate, pursuant to Section 2.3.2(b)(i) or 2.3.2(b)(iii), as
applicable, and using the Coding System. Subject to the availability of an
Antigen on an exclusive basis as determined in accordance with Section 3.2.2(a)
hereof, if Medarex (on behalf of itself, a Medarex Affiliate, or Third Party)
selects such Antigen for a HuMAb Project, or otherwise elects to exercise, or
grant to one or more Medarex Affiliates or Third Parties, rights to use the
Medarex Technology and/or Medarex Mice to Exploit Antibodies (and/or Antibody
Products) against an Antigen as contemplated herein on an Antigen-exclusive
basis pursuant to this Section 4.1, it shall pay to Kirin the applicable Antigen
Exclusivity Fee pursuant to Section 10.4.3 hereof, if any, within [*] days after
such exercise or grant, as the case may be.
4.2 Reservation Licenses.
4.2.1 In General. Subject to the availability of an Antigen as
determined in accordance with Article III hereof, each Party may exercise its
rights under a Reservation License with respect to such Antigen on behalf of
itself, or with the intent of granting a sublicense under such Reservation
License, as follows.
4.2.2 Exercise by Medarex of a Reservation License. In the
event that Medarex desires to exercise its rights under a Reservation License
with respect to an Antigen and such Antigen is available to Medarex for the
exercise of such rights pursuant to the terms and conditions of Section 3.2.2
hereof, Medarex shall provide notice to Kirin pursuant to this Section 4.2.2 and
indicate that it is exercising its rights under a Reservation License (which
notice need not identify the Antigen for which the rights are being exercised
(except in the case of In-House Projects, in which case Medarex shall disclose
the identity of the Antigen(s) to Kirin on Medarex's In-House Project List), but
shall in any event
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-48-
identify the code assigned by Medarex to such Antigen pursuant to the Coding
System). With respect to each Antigen for which Medarex exercises its rights
under a Reservation License, Medarex shall (a) add such Antigen to the Medarex
Non-Exclusive Antigen List (or, if such Antigen is a ROFR Target, Medarex shall
add such Antigen to the Shared Exclusive Antigen List or the Medarex Restricted
Antigen List, as the case may be) pursuant to Section 2.3.2(b)(i) hereof, and
(b) pay to Kirin the Reservation License Fee, if any, pursuant to Section 10.2
hereof.
4.2.3 Exercise by Kirin of a Reservation License. In the event
that Kirin desires to exercise its rights under a Reservation License with
respect to an Antigen, it shall make an Antigen Availability Inquiry to Medarex
in accordance with the terms of Section 3.3.1(a) hereof, and as part of such
inquiry shall notify Medarex that Kirin desires to exercise its rights under a
Reservation License with respect to the Antigen that is the subject of the
inquiry (and whether, if available for the exercise of such a License, upon such
exercise by Kirin such Antigen would be a ROFR Target for which Refusal Rights
are held by a Third Party). Within the time in which Medarex is required to
respond to such Antigen Availability Inquiry pursuant to Section 3.3.1(c)
hereof, Medarex shall inform Kirin whether Kirin may exercise its rights under a
Reservation License with respect to such Antigen, i.e., whether rights with
respect to such Antigen are available on a non-exclusive basis (or, if such
Antigen upon such exercise by Kirin would be a ROFR Target, whether rights are
available on an exclusive basis, pursuant to Section 3.3.2(a) hereof). In the
event that Medarex notifies Kirin in writing that such Antigen is available to
Kirin for the exercise of its rights under such Reservation License, Kirin shall
be deemed to have exercised its rights under a Reservation License for such
Antigen as of the date on which Medarex provides such notice to Kirin. In the
event that Medarex indicates that the Antigen is not available to Kirin for the
exercise of rights under a Reservation License with respect to such Antigen,
Kirin may not exercise its rights under such Reservation License with respect to
such Antigen, unless and until it determines that such Antigen is available
pursuant to a subsequent Antigen Availability Inquiry and complies with the
terms and conditions of this Section 4.2.3. With respect to each Antigen for
which Kirin exercises its rights under a Reservation License, Kirin shall (a)
add such Antigen to the Kirin Non-Exclusive Antigen List pursuant to Section
2.3.2(b)(ii) hereof (except that if the Antigen is a ROFR Target, Medarex shall
add such Antigen to the Shared Exclusive Antigen List), and (b) pay to Medarex a
Reservation License Fee, if any, pursuant to Section 10.2 hereof.
4.2.4 Renewal of Reservation License. In the event that a
Party has exercised a Reservation License with respect to an Antigen pursuant to
Section 4.2.2 or 4.2.3 hereof, as applicable, such Party shall be permitted to
renew such License for [*] Reservation License Periods. In the event that a
Party desires to renew a Reservation License, it shall provide notice to the
other Party [*] days prior to the expiration of the then-applicable Reservation
License Period, in which event the notifying Party shall be deemed to have
renewed the Reservation License as of the date the prior Reservation License
period expires. The Party renewing a Reservation License pursuant to this
Section 4.2.4 shall be required to pay to the other Party, with respect to each
such renewal the additional Reservation License Fee, if any, for each renewal
period pursuant to Section 10.2 hereof.
4.2.5 Covenant of Reservation License Holder. Each Party
covenants to the other Party that with respect to any Antigen that is the
subject of a Reservation License under which such Party has exercised rights
pursuant to Section 4.2.2 or Section 4.2.3 hereof, as applicable, such Party
shall not file an IND in relation to an Antibody raised against and with
affinity for the Reservation Target (or in relation to any Antibody Product
containing such Antibody or Antibody Material related thereto) without first
exercising its rights under a Commercial License for such Reservation Target
pursuant to Section 4.3 hereof and paying the Commercial License Fee, if any,
pursuant to Section 10.3 hereof. Each Party further covenants to the other Party
with respect to any such Antigen that, if after the Effective Date it enters
into a Project Agreement in connection with a Partner Project and/or In-House
Project, as the case
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-49-
may be, with a Partner and/or an In-House Collaborator, as the case may be, in
which Project Agreement it grants to such Partner and/or In-House Collaborator a
sublicense under a License granted to it in this Agreement, such Project
Agreement, shall provide that the Partner and/or In-House Collaborator, as the
case may be, may not in the exercise of its rights under such sublicense file an
IND in relation to any Antibody raised against and with affinity for such
Antigen (or any Antibody Product containing such Antibody or Antibody Material
related thereto) without first obtaining from such Party a sublicense under a
Commercial License with respect to such Antibody.
4.3 Commercial Licenses.
4.3.1 Exercise In General. Subject to the availability of
Antibodies and Antigens as determined in accordance with Article III hereof,
each Party may exercise its rights under a Commercial License with respect to an
Antigen pursuant to this Section 4.3 on behalf of itself, or with the intent of
granting a sublicense under the Commercial License (subject to Section 8.5.1(d)
hereof) as follows.
4.3.2 Commercial License Rights of Reservation License
Holders. The holder of a Reservation License with respect to a Reservation
Target shall, by virtue of holding such Reservation License and subject to the
terms and conditions of this Agreement, including this Section 4.3.2, have a
right to exercise its rights under an Antigen Non-Exclusive Commercial License
with respect to such Reservation Target, and if such Reservation Target is
available on an exclusive or semi-exclusive basis as determined in accordance
with Section 3.2.2 or 3.3.2 hereof, as applicable, it shall have a right to
exercise an Antigen Exclusive Commercial License or an Antigen Semi-Exclusive
Commercial License with respect to such Antigen. The exercise requirements of
this Section 4.3.2, in lieu of the exercise requirements of Sections 4.3.3 and
4.3.4 hereof, shall apply in the case of a Reservation Target. The Party holding
a Reservation License with respect to a Reservation Target may exercise its
rights under a Commercial License by delivering to the other Party, at any time
during the Reservation License Period, a written notice indicating its desire to
exercise a Commercial License, which notice shall specify the type of License
under which it desires to exercise such rights (i.e., Antigen Exclusive
Commercial License, an Antigen Semi-Exclusive Commercial License or Antigen
Non-Exclusive Commercial License) and indicate whether such License relates to
an In-House Project or Partner Project. If the Party holding a Reservation
License with respect to a Target indicates that it desires to exercise an
Antigen Exclusive Commercial License, an Antigen Semi-Exclusive Commercial
License and/or an Antigen Non-Exclusive Commercial License with respect to such
Target, and such Target is available for the exercise of such license (as
determined in accordance with Article III hereof), such Party shall be deemed to
have exercised such Commercial License, as of the date the Target is determined
by Medarex to be so available, in the case of any exercise by Medarex, and as of
the date that Medarex notifies Kirin in writing that such Target is so
available, in the case of any exercise by Kirin. If the Party holding a
Reservation License indicates that it desires to exercise its rights under an
Antigen Exclusive Commercial License or an Antigen Semi-Exclusive License with
respect to the Reservation Target, and such Target is not available for exercise
of the requested license, the requesting Party will be deemed not to have
exercised any Commercial License with respect to such Target unless such Party
expressly indicates in the notice provided pursuant to this Section 4.3.2 that
it desires to exercise its rights under another type of Commercial License in
the event that the first requested Commercial License is unavailable (and such
other Commercial License is available), in which case such Party shall be deemed
to have exercised its rights under such other Commercial License with respect to
such Target. In the event that a Party exercises its rights under an Antigen
Exclusive Commercial License or an Antigen Semi-Exclusive Commercial License,
such Target shall be removed from the Medarex Non-Exclusive Antigen List or the
Kirin Non-Exclusive Antigen List, as applicable, and Medarex shall add such
Target to the Shared Exclusive Antigen List or the Medarex Restricted Antigen
List pursuant to Section 2.3.2(b)(i) or 2.3.2(b)(ii) hereof, as applicable. The
Party exercising its rights under the Commercial License shall pay
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-50-
to the other Party a Commercial License Fee, if any, pursuant to Section 10.3
hereof, and, if the License exercised is an Antigen Exclusive Commercial License
or an Antigen Semi-Exclusive Commercial License, an Antigen Exclusivity Fee, if
any, pursuant to Section 10.4 hereof.
4.3.3 Exercise By Medarex of a Commercial License.
(a) Available Antigens. In the event that Medarex desires to
exercise its rights under either an Antigen Non-Exclusive Commercial License,
Antigen Exclusive Commercial License, or Antigen Semi-Exclusive Commercial
License with respect to an Antigen (other than a Medarex Reservation Target with
respect to which Medarex has the right to exercise a Commercial License pursuant
to Section 4.3.2 hereof) and determines that such Antigen is available to
Medarex for the exercise of rights under such License pursuant to the terms and
conditions of Section 3.2.2(a) or 3.2.2(b) hereof, Medarex shall provide notice
to Kirin pursuant to this Section 4.3.3(a) and indicate that Medarex is
exercising its rights under an Antigen Exclusive Commercial License, an Antigen
Non-Exclusive Commercial License, or an Antigen Semi-Exclusive Commercial
License, as the case may be, and whether such License is exercised in connection
with an In-House Project or a Partner Project. With respect to each Antigen for
which Medarex exercises a Commercial License, Medarex shall (a) add, pursuant to
Section 2.3.2(b)(i) hereof, such Antigen to the Medarex Non-Exclusive Antigen
List, if Medarex has exercised its rights under an Antigen Non-Exclusive
Commercial License, or the Shared Exclusive Antigen List or the Medarex
Restricted Antigen List, as applicable, if Medarex has exercised its rights
under an Antigen Exclusive Commercial License or an Antigen Semi-Exclusive
Commercial License, and (b) pay to Kirin a Commercial License Fee, if any,
pursuant to Section 10.3 hereof, and if such License exercised is an Antigen
Exclusive Commercial License or an Antigen Semi-Exclusive Commercial License, an
Antigen Exclusivity Fee, if any, pursuant to Section 10.4 hereof.
(b) Unavailable Antigen. In the event that Medarex desires to
exercise its rights under an Antigen Exclusive Commercial License, Antigen
Semi-Exclusive Commercial License, or Antigen Non-Exclusive Commercial License
with respect to an Antigen (other than a Medarex Reservation Target) and
determines that such Antigen is not available to Medarex for the exercise of
rights under such License pursuant to the terms and conditions of Section 3.2.3
hereof, Medarex may not exercise its rights under a Commercial License with
respect to such Antigen, unless and until it subsequently determines such
Antigen is available pursuant to a subsequent inquiry pursuant to Section 3.2.1
hereof.
(c) ROFR Targets Granted by Medarex. In the event that Medarex
desires to exercise or grant rights with respect to an Antigen pursuant to
Section 4.1 hereof on behalf of Medarex, a Medarex Affiliate or a Third Party,
or to exercise its rights under a Commercial License on behalf of Medarex, a
Medarex Partner or a Medarex In-House Collaborator with respect to an Antigen
and, pursuant to Section 3.2 hereof, has knowledge that such Antigen is a ROFR
Target that is the subject of Refusal Rights granted by Medarex to a Third
Party, Medarex shall notify the ROFR Party that a Person has requested rights
with respect to the Antigen.
(i) In the event that the ROFR Party exercises its
Refusal Rights with respect to such Antigen by providing written notice thereof
to Medarex in a timely manner, as provided under the agreement that provides for
Refusal Rights with respect to such Antigen, Medarex shall not exercise or grant
any rights with respect to the Antigen (other than on behalf of the ROFR Party)
unless and until the Antigen is subsequently determined to be available as part
of a subsequent availability inquiry by Medarex pursuant to the terms and
conditions of Section 3.2 hereof.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-51-
(ii) In the event that the ROFR Party, within the permitted
time period for exercising Refusal Rights, declines to exercise such Refusal
Rights, but elects pursuant to the agreement that provides for such Refusal
Rights and/or with Medarex's consent to exercise or retain certain Antigen
non-exclusive rights (including an option) with respect to such Antigen, Medarex
may grant or exercise rights pursuant to Section 4.1 hereof or exercise rights
under a Medarex Antigen Semi-Exclusive Commercial License or a Medarex Antigen
Non-Exclusive Commercial License, as the case may be, with respect to such
Antigen. In the event that Medarex elects to grant or exercise any such rights
with respect to such Antigen, and to the extent consistent with the rights
retained or exercised by the ROFR Party with respect to such Antigen, Medarex
shall provide to Kirin notice of Medarex's exercise or grant of rights with
respect to the Antigen, as the case may be, which notice shall specify the
rights being granted or exercised by Medarex, and Medarex shall be deemed to
have granted or exercised such rights pursuant to Section 4.3.3(f) hereof.
(iii) In the event that the ROFR Party fails or declines to
exercise its Refusal Rights in a timely manner under the agreement that provides
for Refusal Rights with respect to such Antigen (and does not otherwise elect to
retain or exercise rights with respect to such Antigen in accordance with the
preceding paragraph), then pursuant to Section 4.3.3(f) hereof Medarex shall be
deemed to have exercised or granted rights with respect to such Antigen.
In the event that the ROFR Party, in response to the notice from Medarex
pursuant to the first sentence of this Section 4.3.3(c), provides written notice
to Medarex that it is exercising its Refusal Rights with respect to an Antigen,
or the ROFR Party elects to obtain a license to one or more Antibody(ies) raised
against such Antigen, as the case may be, and such exercise by the ROFR Party
will result in the ROFR Party obtaining from Medarex a sublicense under the
license granted by Kirin to Medarex in Section 8.3.6 hereof, or under a License,
Medarex shall be deemed to have exercised such license on behalf of the ROFR
Party as of the date on which Medarex provides notice to Kirin of the ROFR
Party's exercise of its Refusal Rights or other election, and all of the terms,
conditions and obligations that apply to such License under this Agreement shall
apply, including, without limitation, listing obligations under Article II
hereof and payment obligations under Article X hereof.
(d) ROFR Targets Granted by Kirin. In the event that Medarex desires
to exercise or grant rights with respect to an Antigen pursuant to Section 4.1
hereof on behalf of a Third Party, or to exercise its rights under a Commercial
License on behalf of a Medarex Partner or a Medarex In-House Collaborator with
respect to an Antigen and, pursuant to Section 3.2 hereof, has knowledge that
such Antigen is a ROFR Target that is the subject of Refusal Rights granted by
Kirin to a Third Party, Medarex shall provide a written notice thereof to Kirin.
Such notice shall specify that Medarex desires to either grant rights to such
Third Party with respect to the Antigen on an Antigen-exclusive basis pursuant
to Section 4.1 hereof or to exercise rights under a Medarex Antigen Exclusive
Commercial License with respect to the Antigen. Additionally, Medarex shall
provide to Kirin a redacted copy of such Third Party's written request for a
license with respect to the Antigen. Upon receipt of such notice from Medarex,
Kirin shall promptly, but in any event within [*] days (x) notify the ROFR Party
that a Third Party has requested exclusive rights with respect to the Antigen,
and (y) provide a copy of such notice to Medarex.
(i) In the event that the ROFR Party exercises its Refusal
Rights with respect to such Antigen by providing written notice thereof to Kirin
within [*] days after the date on which such ROFR Party receives from Kirin the
notice required pursuant to clause (x) of the previous paragraph (in which case
Kirin shall promptly, but in any event within [*] days after the receipt of such
ROFR Party's notice, provide a copy thereof to Medarex), then (A) Kirin shall be
deemed to have exercised a Kirin Antigen Exclusive Commercial License with
respect to such Antigen on behalf of the
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-52-
ROFR Party as of the date on which Kirin provides notice to Medarex of the ROFR
Party's exercise of its Refusal Rights, and all of the terms, conditions and
obligations that apply to such Commercial License under this Agreement shall
apply, including, without limitation, listing obligations under Article II
hereof and payment obligations under Article X hereof, and (B) Medarex shall not
exercise or grant any rights with respect to the Antigen unless and until the
Antigen is subsequently determined to be available as part of a subsequent
availability inquiry by Medarex pursuant to the terms and conditions of Section
3.2 hereof.
(ii) In the event that the ROFR Party, within [*] days from the
date on which the ROFR Party receives notice from Kirin that a Third Party has
requested exclusive rights with respect to the Antigen, declines to exercise
such Refusal Rights, but elects pursuant to the agreement that provides for such
Refusal Rights and/or with Kirin's consent to exercise or retain certain Antigen
non-exclusive rights (including an option) with respect to such Antigen, Kirin
shall promptly, but in any event within [*] days after such ROFR Party's
election to exercise or retain such rights, notify Medarex thereof in writing
and specify the extent to which such Antigen is available for Medarex's exercise
or grant of rights. In such event, (A) if the ROFR Party elected to obtain a
sublicense under a Kirin Antigen Non-Exclusive License, Kirin shall be deemed to
have exercised such license on behalf of the ROFR Party as of the date on which
Kirin provides notice to Medarex of the ROFR Party's election to exercise such
sublicense, and (B) to the extent consistent with the rights retained or
exercised by the ROFR Party, Medarex may grant or exercise such rights pursuant
to Section 4.1 hereof or under a Medarex Antigen Semi-Exclusive Commercial
License or a Medarex Antigen Non-Exclusive Commercial License, as the case may
be, with respect to such Antigen. In the event that Medarex elects to grant or
exercise any such rights with respect to such Antigen, Medarex shall provide to
Kirin promptly, but in any event within [*] days after the receipt by Medarex of
the notice from Kirin regarding the ROFR Party's election pursuant to this
Section 4.3.3(d)(ii), notice of Medarex's election (specifying the rights being
granted or exercised by Medarex), in which case, Medarex shall be deemed to have
granted or exercised such rights pursuant to Section 4.3.3(f) hereof.
(iii) In the event that the ROFR Party fails or declines to
exercise its Refusal Rights with respect to such Antigen within [*] days after
receiving notice from Kirin that a Third Party has requested exclusive rights
with respect to the Antigen (and does not otherwise elect to retain or exercise
rights with respect to such Antigen in accordance with the preceding paragraph),
then Kirin promptly, but in any event within [*] days after receipt by Kirin of
a notice from such ROFR Party declining to exercise its ROFR rights, or the
expiration of such [*] day period, whichever is sooner, shall notify Medarex in
writing that such Antigen is available for Medarex's exercise or grant of
rights. In such event, (A) Kirin's Reservation License on behalf of the ROFR
Party with respect to such Antigen shall terminate and Kirin shall have no
further license or other rights with respect to the Antigen unless and until it
is later determined to be available pursuant to a subsequent Antigen
Availability Inquiry, and (B) pursuant to Section 4.3.3(f) hereof Medarex shall
be deemed to have exercised or granted rights with respect to such Antigen.
(e) ROFR Targets Held by Kirin. In the event that Medarex desires to
exercise or grant rights with respect to an Antigen pursuant to Section 4.1
hereof on behalf of a Third Party, or to exercise its rights under a Commercial
License on behalf of a Medarex Partner or Medarex In-House Collaborator with
respect to an Antigen and, pursuant to Section 3.2 hereof, has knowledge that
such Antigen is a ROFR Target for which Kirin holds Refusal Rights, Medarex
shall provide a written notice thereof to Kirin. Such notice shall specify that
Medarex desires to either grant rights to such Third Party with respect to the
Antigen on an Antigen non-exclusive or Antigen-exclusive basis pursuant to
Section 4.1 hereof or to exercise rights under a Medarex Antigen Exclusive
Commercial License, a Medarex Antigen Semi-Exclusive Commercial License or a
Medarex Antigen Non-Exclusive
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-53-
Commercial License with respect to the Antigen. Additionally, Medarex shall
provide to Kirin a redacted copy of such Third Party's written request for a
license with respect to the Antigen.
(i) In the event that Kirin exercises its Refusal Rights with
respect to such Antigen by providing written notice of such exercise to Medarex
within [*] days after receiving Medarex's notice under this Section 4.3.3(e),
Kirin shall be deemed to have exercised a Kirin Antigen Exclusive Commercial
License with respect to such Antigen as of the date of such notice and Medarex
shall not exercise or grant any rights with respect to the Antigen unless and
until the Antigen is subsequently determined to be available as part of a
subsequent availability inquiry by Medarex pursuant to the terms and conditions
of Section 3.2 hereof.
(ii) In the event that Kirin declines to exercise such Refusal
Rights, but elects to retain a Reservation License with respect to such Antigen,
Kirin shall provide written notice thereof to Medarex within [*] days after
receiving Medarex's notice under this Section 4.3.3(e). In the event that Kirin
timely provides such notice to Medarex, then (A) Kirin shall retain such
Reservation License, but as of the date of such notice shall no longer have
Refusal Rights with respect to such Antigen, and (B) Medarex may exercise or
grant rights consistent with the rights exercised by Kirin with respect to such
Antigen pursuant to Section 4.1 hereof, or under a Medarex Antigen
Semi-Exclusive Commercial License or a Medarex Antigen Non-Exclusive Commercial
License. In the event that Medarex elects to grant or exercise any such rights
with respect to such Antigen, Medarex shall provide to Kirin promptly, but in
any event within [*] days after the receipt by Medarex of the notice from Kirin
regarding its exercise of a Kirin Antigen Non-Exclusive Commercial License
pursuant to this Section 4.3.3(e)(ii), notice of Medarex's election (specifying
the rights being granted or exercised by Medarex), in which case, Medarex shall
be deemed to have granted or exercised such rights pursuant to Section 4.3.3(f)
hereof.
(iii) In the event that Kirin fails or declines to exercise its
Refusal Rights with respect to such Antigen within [*] days after receiving
Medarex's notice under this Section 4.3.3(e) (and does not elect to retain or
exercise rights with respect to such Antigen in accordance with the preceding
paragraph), then (A) pursuant to Section 4.3.3(f) hereof Medarex shall be deemed
to have exercised or granted exclusive rights with respect to such Antigen
pursuant to Section 4.1 hereof, or to exercise exclusive rights under a
Commercial License with respect to such Antigen, and (B) Kirin's Reservation
License and its Refusal Rights with respect to such Antigen shall terminate and
Kirin shall have no further license or other rights with respect to the Antigen
unless and until it is later determined to be available pursuant to a subsequent
Antigen Availability Inquiry.
(f) Medarex's Exercise of Rights to the Antigen That Was Subject to
Refusal Rights. In the event that Medarex has provided notice to Kirin pursuant
to Section 4.3.3(c), (d), or (e) hereof that Medarex, any of its Affiliates, or
any Third Party desires to obtain or exercise a license with respect to a
particular Antigen, then as of the date on which Medarex has knowledge, pursuant
to Section 4.3.3(c), (d) or (e) above, that the ROFR Party failed or declined to
exercise timely its Refusal Rights with respect to such Antigen, or that the
ROFR Party has declined to exercise Refusal Rights with respect to an Antigen
but has timely elected to exercise or retain certain Antigen non-exclusive
rights with respect to an Antibody(ies) against such Antigen, (and to the extent
consistent with the ROFR Party's rights with respect to such Antigen, if any),
Medarex shall be automatically deemed to have exercised or granted rights with
respect to such Antigen pursuant to Section 4.1 hereof, or to have exercised its
rights under a Medarex Antigen Exclusive Commercial License, Medarex Antigen
Non-Exclusive Commercial License, or Medarex Antigen Semi-Exclusive Commercial
License, as the case may be, with respect to a particular Antigen, as specified
in the notices required under Sections 4.3.3(c), (d) or (e) above, and all of
the terms, conditions and obligations that apply to such specified rights under
this Agreement shall apply,
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchan
ge Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-54-
including, without limitation, listing obligations under Article II hereof and
payment obligations, if any, under Article X hereof.
4.3.4 Exercise by Kirin of a Commercial License.
(a) Notice. In the event that Kirin desires to exercise its
rights under a Commercial License with respect to an Antigen (other than a Kirin
Reservation Target with respect to which Kirin has the right to exercise a
Commercial License pursuant to Section 4.3.2 hereof), it shall make an Antigen
Availability Inquiry to Medarex in accordance with the terms of Section 3.3.1(a)
hereof, and as part of such inquiry shall notify Medarex (i) that Kirin desires
to exercise its rights under an Antigen Exclusive Commercial License, Antigen
Non-Exclusive Commercial License, or Antigen Semi-Exclusive Commercial License,
as the case may be, with respect to the Antigen that is the subject of the
inquiry, and (ii) whether the License with respect to which Kirin desires to
exercise rights relates to an In-House Project or a Partner Project. Within the
time in which Medarex is required to respond to such Antigen Availability
Inquiry pursuant to Section 3.3.1(c) hereof, Medarex shall inform Kirin whether
such Antigen is available to Kirin for Kirin's exercise of its rights under the
applicable License, as determined in accordance with Sections 3.3.2, 3.3.3 and
3.3.4 hereof.
(b) Available Antigen. In the event that Medarex notifies Kirin
pursuant to Section 4.3.4(a) hereof that the Antigen that is the subject of an
Antigen Availability Inquiry is available to Kirin for the exercise of the
License designated in the notice provided by Kirin to Medarex pursuant to
Section 4.3.4(a) hereof, Kirin shall be deemed to have exercised a Commercial
License for such Antigen as of the date Medarex provides such notice to Kirin.
With respect to each Antigen for which Kirin has exercised its rights under an
Antigen Non-Exclusive Commercial License, Kirin shall add such Antigen to the
Kirin Non-Exclusive Antigen List pursuant to Section 2.3.2(b)(ii) hereof. With
respect to each Antigen for which Kirin has exercised its rights under an
Antigen Exclusive Commercial License or an Antigen Semi-Exclusive Commercial
License, Medarex shall add such Antigen to the Shared Exclusive Antigen List
pursuant to Section 2.3.2(b)(ii) hereof. Kirin shall pay to Medarex a Commercial
License Fee, if any, for each Antigen with respect to which Kirin has exercised
its rights under a Commercial License pursuant to Section 10.3 hereof, and if
the License is an Antigen Exclusive Commercial License or Antigen Semi-Exclusive
Commercial License, an Antigen Exclusivity Fee, if any, pursuant to Section 10.4
hereof.
(c) Unavailable Antigen. In the event that Medarex notifies
Kirin pursuant to Section 4.3.4(a) hereof that the Antigen that is the subject
of an Antigen Availability Inquiry is not available to Kirin for the exercise of
a Commercial License designated in the notice provided by Kirin pursuant to
Section 4.3.4(a) hereof, Kirin may not exercise its rights under a Commercial
License with respect to such Antigen, unless and until it subsequently
determines such Antigen is available pursuant to a subsequent Antigen
Availability Inquiry and complies with the terms and conditions of this Section
4.3.4. If Kirin has given notice to Medarex of Kirin's desire to exercise its
rights under an Antigen Exclusive Commercial License and such Antigen is not
available for use on an exclusive basis, Kirin may exercise its rights under an
Antigen Semi-Exclusive Commercial License or Antigen Non-Exclusive Commercial
License with respect to such Antigen upon submitting to Medarex a subsequent
Antigen Availability Inquiry and a revised notice indicating that Kirin desires
to exercise its rights under such a License with respect to such Antigen,
provided that Medarex confirms its availability for such exercise upon receipt
of such subsequent inquiry and revised notice pursuant to the terms of Article
III hereof.
(d) ROFR Targets Granted By Medarex. In the event that Medarex
notifies Kirin pursuant to Section 4.3.4(a) hereof that the Antigen that is the
subject of an Antigen Availability
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-55-
Inquiry and a request by Kirin to exercise rights under a Commercial License is
a ROFR Target with respect to which Medarex has granted Refusal Rights to a
Third Party, Medarex shall promptly, but in any event within [*] days, (x)
notify the ROFR Party that a Person has requested rights with respect to the
Antigen, (y) provide a redacted copy of the notice to Kirin, and (z) to the
extent that Medarex is permitted to do so consistent with its confidentiality
obligations to the ROFR Party, as determined by Medarex in Medarex's reasonable
discretion, disclose to Kirin the ROFR Party's deadline for exercising its
Refusal Rights for the Antigen if such deadline is greater than [*] days from
notice provided by Medarex that a Person desires rights to such Antigen;
provided, however, that in the event that the agreement that provides Refusal
Rights with respect to such Antigen provides that such rights are triggered by a
Third Party's request for exclusive commercial rights with respect to such
Antigen and/or Antibodies against such Antigen, Medarex shall have no obligation
to provide the ROFR Party notice in accordance with clause (x) of this Section
(or to provide a copy of such notice to Kirin) unless Kirin has requested a
Kirin Antigen Exclusive Commercial License with respect to such Antigen. In any
instance where Kirin has requested a Commercial License with respect to a ROFR
Target that is subject to this Section 4.3.4(d) and Medarex determines due to
the nature of the rights requested by Kirin that Medarex does not have an
obligation to notify the ROFR Party of such request consistent with the
foregoing sentence, Medarex shall notify Kirin in writing within [*] days after
the receipt by Medarex of Kirin's request for a Commercial License that Medarex
has received its request for a Commercial License with respect to the Antigen,
the Antigen is a ROFR Target, and Medarex has no obligation to notify the ROFR
Party of such request.
(i) In the event that Medarex provides notice to the ROFR
Party in accordance with the first paragraph of this Section 4.3.4(d) and the
ROFR Party exercises its Refusal Rights with respect to the Antigen by providing
timely written notice thereof to Medarex, Medarex shall promptly, but in any
event within [*] days after such ROFR Party's notice, provide written notice
thereof to Kirin, and Kirin shall not exercise or grant rights under any
Commercial License with respect to such Antigen unless and until the Antigen is
determined to be available in response to a subsequent Antigen Availability
Inquiry.
(ii) In the event that Medarex provides notice to the ROFR
Party in accordance with the first paragraph of this Section 4.3.4(d) and the
ROFR Party, within the time period for exercising Refusal Rights declines to
exercise such Refusal Rights, but elects pursuant to the agreement that provides
for such Refusal Rights and/or with Medarex's consent to exercise or retain
certain Antigen non-exclusive rights (including an option) with respect to such
Antigen, Medarex shall promptly, but in any event within [*] days after the ROFR
Party's election to exercise or retain such rights, notify Kirin thereof, and to
the extent consistent with the rights retained or exercised by the ROFR Party,
Kirin may exercise a Kirin Antigen Semi-Exclusive Commercial License or a Kirin
Antigen Non-Exclusive Commercial License with respect to such Antigen. In the
event that Kirin elects to grant or exercise any such rights with respect to
such Antigen, Kirin shall provide to Medarex promptly, but in any event within
[*] days after the receipt by Kirin of the notice from Medarex regarding the
ROFR Party's election pursuant to this Section 4.3.4(d)(ii), notice of Kirin's
election (specifying the license under which rights are being exercised by
Kirin), in which case, Kirin shall be deemed to have granted or exercised such
rights pursuant to Section 4.3.3(f) hereof.
(iii) In the event that Medarex provides notice to the ROFR
Party in accordance with the first paragraph of this Section 4.3.4(d), and the
ROFR Party fails or declines to exercise its Refusal Rights for the Antigen
within a timely manner as provided under the agreement that provides for Refusal
Rights with respect to such Antigen (and does not otherwise elect to retain or
exercise rights with respect to such Antigen in accordance with the preceding
paragraph), then Medarex shall promptly, but in any event within [*] days after
the expiration of the period in which such ROFR Party has a right to exercise
Refusal Rights, notify Kirin in writing that the Antigen is available for
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-56-
Kirin's exercise of a Commercial License, and pursuant to Section 4.3.4(f) Kirin
shall be deemed to have exercised a Kirin Antigen Exclusive Commercial License
or a Kirin Antigen Non-Exclusive Commercial License, as specified in the notice
provided by Kirin to Medarex in connection with the Antigen Availability
Inquiry.
In the event that the ROFR Party, in response to the notice from Medarex
pursuant to the first sentence of this Section 4.3.4(d), provides written notice
to Medarex that it is exercising its Refusal Rights with respect to an Antigen,
or the ROFR Party elects to obtain a license to one or more Antibody(ies) raised
against and with affinity for such Antigen, as the case may be, and such
exercise by the ROFR Party will result in such ROFR Party obtaining from Medarex
a sublicense under the license granted by Kirin to Medarex in Section 8.3.6 or
under a Medarex Antigen Exclusive Commercial License or a Medarex Antigen
Non-Exclusive Commercial License, Medarex shall be deemed to have exercised such
license on behalf of the ROFR Party as of the date on which Medarex provides
notice to Kirin of the ROFR Party's exercise of its Refusal Rights or other
election, and all of the terms, conditions and obligations that apply to such
Commercial License under this Agreement shall apply, including, without
limitation, listing obligations under Article II hereof and payment obligations
under Article X hereof.
(e) ROFR Targets Granted by Kirin. In the event that Kirin desires to
exercise rights under a Commercial License with respect to an Antigen that is a
ROFR Target with respect to which Kirin has granted Refusal Rights to a Third
Party, Kirin shall notify the ROFR Party that a Person has requested rights with
respect to the Antigen, and Kirin shall also promptly provide a copy of such
notice to Medarex and indicate the type of Commercial License under which Kirin
desires to exercise rights with respect to such Antigen.
(i) In the event that within [*] days after the date on which
such ROFR Party receives from Kirin written notice that a Person desires rights
with respect to the Antigen the ROFR Party exercises its Refusal Rights with
respect to such Antigen by providing written notice thereof to Kirin, Kirin
shall within [*] days after the receipt of such notice provide written notice
thereof to Medarex, together with a copy of the ROFR Party's notice, and Kirin
shall not exercise or grant its rights under any Commercial License (other than
on behalf of the ROFR Party) with respect to such Antigen unless and until the
Antigen is determined to be available in response to a subsequent Antigen
Availability Inquiry.
(ii) In the event that the ROFR Party, within [*] days after
the date on which such ROFR Party receives from Kirin written notice that a
Person desires rights with respect to the Antigen, declines to exercise such
Refusal Rights, but elects pursuant to the agreement that provides for such
Refusal Rights and/or with Kirin's consent to exercise or retain certain Antigen
non-exclusive rights (including an option) with respect to such Antigen, Kirin
shall promptly, but in any event within [*] days after the ROFR Party's election
to exercise or retain such rights, notify Medarex thereof. To the extent
consistent with the rights retained or exercised by the ROFR Party, Kirin may
exercise such Kirin Antigen Semi-Exclusive Commercial License or Kirin Antigen
Non-Exclusive Commercial License, as the case may be, with respect to such
Antigen. In the event that Kirin elects to grant or exercise any such rights
with respect to such Antigen, Kirin shall notify Medarex, as part of the notice
provided by Kirin to Medarex with regard to such ROFR Party's election pursuant
to this Section 4.3.4(e)(ii), of Kirin's election to exercise or grant of rights
with respect to the Antigen, as the case may be, which notice shall specify the
rights being exercised by Kirin, in which case Kirin shall be deemed to have
granted or exercised such rights pursuant to Section 4.3.3(f) hereof.
(iii) In the event that the ROFR Party fails or declines to
exercise its Refusal Rights for the Antigen within [*] days after receipt
thereof (and does not otherwise elect to retain
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-57-
or exercise rights with respect to such Antigen in accordance with the preceding
paragraph), then Kirin shall promptly, but in any event within [*] days after
the expiration of such [*] day period, notify Medarex thereof in writing that
such Antigen is available for Kirin's exercise or grant of a Commercial License
and Kirin shall be deemed to have exercised a Kirin Antigen Exclusive Commercial
License with respect to the Antigen pursuant to Section 4.3.4(f) hereof.
In the event that the ROFR Party, in response to the notice from Kirin pursuant
to the first sentence of this Section 4.3.4(e), provides written notice to Kirin
that it is exercising its Refusal Rights with respect to an Antigen, or elects
in accordance with this Section 4.3.4(e) to obtain a sublicense under a Kirin
Antigen Non-Exclusive Commercial License, as the case may be, Kirin shall be
deemed to have exercised a Kirin Antigen Exclusive Commercial License or a Kirin
Antigen Non-Exclusive Commercial License, as the case may be, on behalf of the
ROFR Party as of the date on which Kirin provides notice to Medarex of the ROFR
Party's exercise of its Refusal Rights or other election, and all of the terms,
conditions and obligations that apply to such Commercial License under this
Agreement shall apply, including, without limitation, listing obligations under
Article II hereof and payment obligations under Article X hereof.
(f) Kirin's Exercise of Rights to the Antigen That Was
Subject to Refusal Rights. In the event that Kirin has provided notice to
Medarex pursuant to Section 4.3.4(d) or (e) hereof that Kirin desires to
exercise its rights under a Commercial License with respect to a particular
Antigen, then as of the date on which Medarex provides notice to Kirin pursuant
to Section 4.3.4(d) hereof that the ROFR Party has declined to exercise Refusal
Rights and/or has exercised less than all of the available rights with respect
to such Antigen, in the case of a ROFR Party to which Medarex has granted
Refusal Rights, or the date on which Medarex has received notice from Kirin
pursuant to Section 4.3.4(e) hereof that the ROFR Party has declined to exercise
Refusal Rights and/or has exercised less than all of the available rights with
respect to such Antigen, in the case of a ROFR Party to which Kirin has granted
Refusal Rights, and to the extent consistent with the ROFR Party's rights with
respect to such Antigen, if any, Kirin shall be automatically deemed to have
exercised rights under a Kirin Antigen Exclusive Commercial License, Kirin
Antigen Non-Exclusive Commercial License, or Kirin Antigen Semi-Exclusive
Commercial License with respect to such Antigen, as specified in the Antigen
Availability Inquiry provided by Kirin to Medarex with respect to such Antigen,
and all of the terms, conditions and obligations that apply to such Commercial
License under this Agreement shall apply, including, without limitation, listing
obligations under Article II hereof and payment obligations under Article X
hereof.
4.4 [*].
4.4.1 In General. Each Party (and its Affiliates, Partners and
In-House Collaborators) shall have the right to exercise the licenses and other
rights granted to it under a Commercial License pursuant to Article VIII hereof
only with respect to [*]; provided, however, that in the case of a Special
Licensee, the Special Licensee shall be permitted to exercise a sublicense under
the rights granted to Medarex in Section 8.3.6 hereof with respect to [*]
Antibodies selected by such Special Licensee pursuant to the terms and
conditions of the Special License.
(a) [*]. Within the scope of a Party's Antigen
Non-Exclusive Commercial License with respect to a particular Antigen, a Party
shall have the right to [*], subject to the availability of such [*] as
determined in accordance with Article III hereof, and the terms and conditions
of Section 4.4.2 hereof; provided, however, that a Party shall have the right to
[*], with the prior written consent of the other Party, [*].
(b) [*]. A Party that has exercised its rights under an
Antigen Exclusive Commercial License or an Antigen Semi-Exclusive Commercial
License with respect to a particular
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-58-
Antigen pursuant to Section 8.2.4(a), 8.2.4(c), 8.3.4(a), or 8.3.4(c) hereof, as
applicable, shall have the right to [*] for such License [*], subject to the
availability of such [*] as determined in accordance with Article III hereof and
the terms and conditions of Section 4.4.2 hereof.
4.4.2 [*]. A Party that has exercised its rights under a
Commercial License granted by the other Party herein with respect to a
particular Antigen shall, subject to Section 4.4.1, 4.4.2(a), 4.4.2(b) or
4.4.2(c) hereof, as applicable, be permitted to [*] for such License, as
follows:
(a) By Medarex.
(i) Antibody Identification Notice. If at any time
during the term of a Commercial License granted by Kirin to Medarex, Medarex
desires to [*] for such Commercial License and determines that such
Collaboration Antibody is available to Medarex pursuant to the terms and
conditions of Section 3.4 hereof, Medarex shall provide written notice to Kirin
and indicate that it is [*] (which notice need not identify the Collaboration
Antibody). In the written notice provided by Medarex to Kirin pursuant to this
Section 4.4.2(a)(i), Medarex shall identify the Collaboration Antibody and the
Medarex Commercial Target against which such Collaboration Antibody was raised
by the code assigned by Medarex pursuant to the Coding System and shall not be
required in such notice to identify the Collaboration Antibody by its Antibody
Amino Acid Sequence or the Medarex Commercial Target by a sequence or other
identifying information.
(ii) Antibody Available. Upon the giving of notice
to Kirin pursuant to this Section 4.4.2(a) hereof, such Collaboration Antibody
shall be deemed to be [*]. Medarex shall add each Collaboration Antibody [*] to
the Antibody Sequence List pursuant to Section 2.3.2(b)(iii) hereof.
(iii) Antibody Unavailable. In the event that such
Collaboration Antibody is not available [*], as determined in accordance with
Article III hereof, Medarex may not [*] such Collaboration Antibody [*], unless
and until it subsequently determines such Collaboration Antibody is available,
as determined in accordance with Article III hereof.
(b) By Kirin.
(i) Antibody Identification Notice. If at any time
during the term of a Commercial License granted by Medarex to Kirin, Kirin
desires to [*] for such Commercial License, Kirin shall make an Antibody
Availability Inquiry to Medarex in accordance with the terms of Section 3.5.1
hereof, and as part of such inquiry shall (a) notify Medarex that Kirin desires
to [*], and (b) identify the Kirin Commercial Target against which such Antibody
was raised. Within [*] business days Medarex shall notify Kirin in writing
whether such Collaboration Antibody is available to Kirin, as determined in
accordance with Section 3.5.1 hereof.
(ii) Antibody Available. In the event that Medarex
notifies Kirin that such Collaboration Antibody is available to Kirin [*], such
Collaboration Antibody shall be deemed to be [*] as of the date that Medarex
notifies Kirin of such availability. Medarex shall add each Collaboration
Antibody [*] to the Antibody Sequence List pursuant to Section 2.3.2(b)(iii)
hereof.
(iii) Antibody Unavailable. In the event that Medarex
notifies Kirin that such Collaboration Antibody is not available to Kirin [*],
Kirin may not [*] such Collaboration Antibody [*], unless and until it
subsequently determines such Collaboration Antibody is available in response to
a subsequent Antibody Availability Inquiry pursuant to Section 4.4.2(b).
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-59-
(c) [*] Each Party shall have the right with respect to any
Commercial License granted to it by the other Party to [*] for such Commercial
License with a [*], subject to the availability of the [*]. A Party that desires
to [*] shall provide written notice thereof to the other Party and satisfy the
terms and conditions in Section 4.4.2(a)(ii) or 4.4.2(b)(ii) hereof, as
applicable, governing [*] of a Collaboration Antibody [*]. If the Collaboration
Antibody that such Party seeks to [*] is determined to be available in
accordance with Section 3.4 or 3.5 hereof, as applicable, then in the case of
designations made by Medarex, such [*] shall be deemed to be [*] as of the date
it provides such written notice to Kirin, and in the case of Kirin, such [*]
shall be deemed to be [*] as of the date that Medarex notifies Kirin in writing
of such Collaboration Antibody's availability. From and after the [*] Date, the
[*] that was [*] shall be [*] and the holder of the Commercial License with
respect to [*] shall have no further rights to Exploit or have Exploited such
Collaboration Antibody or Collaboration Products containing such Collaboration
Antibody. Medarex shall [*] from the Antibody Sequence List in accordance with
Section 2.3.3(b) hereof and [*] to the Antibody Sequence List in accordance with
Section 2.3.2(b)(iii) hereof, whereupon the [*] shall no longer constitute a
[*], and such [*] shall be deemed a [*].
ARTICLE V
IN-HOUSE PROJECTS
5.1 In-House Target List.
5.1.1 In-House Target Lists. Each Party shall prepare and maintain
an In-House Target List in accordance with Section 2.2.1 hereof.
5.1.2 [*]. Except as set forth in Section 5.1.3(d)(i) or 5.3.2
hereof, and in accordance with Section 5.5 hereof, each Party, when including
In-House Targets on its respective In-House Target List, shall not include [*].
5.1.3 Designation of Targets on In-House Target List.
(a) Designation of Targets On or After the Effective Date. If
on or after the Effective Date a Party exercises its rights under a Reservation
License pursuant to Section 4.2 hereof or a Commercial License pursuant to
Section 4.3 hereof with respect to an Antigen in connection with an In-House
Project, the Party exercising such rights shall designate such Antigen as an
In-House Target and note the corresponding Designation Date on its In-House
Target List within [*] business days after the exercise of such rights. Such
Target shall remain on such Party's In-House Target List unless and until such
Target is removed therefrom pursuant to Section 5.5 hereof. [*], a Party shall
not exercise a License pursuant to Article IV hereof with respect to an Antigen
in connection with an In-House Project if the exercise of such License and
corresponding designation of such Antigen on such Party's In-House Target List
would cause such Party to [*].
(b) Designation of Targets Before the Effective Date. If prior
to the Effective Date a Party designated one or more Antigens on its In-House
Target List in reliance on the Agreement on Essential Terms for Collaboration,
the Designation Date for each such Target shall be the date on which the
Designating Party added such Target to its In-House Target List (as noted on
such list). Such Target(s) shall remain on such In-House Target List unless and
until such Target is removed therefrom pursuant to Section 5.5 hereof.
(c) Further Designation of Targets as [*] Targets or [*]
Targets. Except in the case of an Antigen selected by a Party as a [*] Target
pursuant to Section 5.3.2 hereof, which Antigen
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-60-
shall be designated as a [*] Target at the time such Antigen is listed on the
In-House Target List, each Party shall be permitted to list an Antigen on its
In-House Target List pursuant to Section 5.1.3(a) or (b) hereof without further
designating at the time of such listing whether the Antigen is a [*] Target or a
[*] Target, provided that such Party further designates such Antigen as a [*]
Target pursuant to Section 5.3 hereof or a [*] Target pursuant to Section 5.2
hereof prior to such Party exercising rights under any Commercial License with
respect to one or more Antibodies raised against and with affinity for such
Target. In addition, at any time after a Party adds an Antigen to its In-House
Target List, such Party shall be permitted to designate such Antigen as an [*]
Target pursuant to Section 5.4 hereof.
(d) Opt-In Process and Effects on the [*]. At any time during
the Term, a Party may in its sole discretion make an Opt-In Offer to the other
Party in accordance with the terms of this Section 5.1.3(d) hereof with respect
to an Antigen on or intended to be added to the offering Party's In-House Target
List.
(i) Opt-In Process With Respect to New Targets. In the
event that the Designating Party desires to have the Non-Designating Party
collaborate on an In-House Project with respect to an Antigen that has not yet
been added to the Designating Party's In-House Target List, regardless of
whether such designation will result in the Designating Party [*], the
Designating Party shall (A) provide written notice to the other Party that it
desires to exercise its rights pursuant to this Section 5.1.3(d)(i) with respect
to such Antigen and simultaneously therewith make an Opt-In Offer to the other
Party with respect to such Antigen, and (B) as of the date of such notice
designate such Antigen as an In-House Target and note the corresponding
Designation Date on its In-House Target List. Within [*] business days after the
Designation Date, subject to Section 5.1.3(d)(iii) hereof, the Designating Party
shall provide to the Non-Designating Party Antigen Evaluation Materials relating
to such Target and thereafter [*] to the Non-Designating Party in its review and
analysis of such materials. The Non-Designating Party shall have [*] days from
the date on which a complete set of the Antigen Evaluation Materials is received
to accept or reject the Designating Party's Opt-In Offer; provided, however, if
at the time that the Designating Party makes an Opt-In Offer to the
Non-Designating Party with respect to an Antigen, the Designating Party's
designation of such Antigen on its In-House Target List will not cause the
Designating Party to [*], the Non-Designating Party may request that the
Designating Party consent to [*] days within which to accept or reject the
Opt-In Offer, [*]. In the event that the Non-Designating Party timely accepts
the Opt-In Offer, the Parties shall negotiate [*] the terms and conditions of a
definitive agreement governing the subject matter of the Opt-In Offer and such
Target shall [*] during the negotiation period. In the event that the Parties
are unable [*] to reach agreement on the terms of a definitive agreement with
respect to the subject matter of the Opt-In Offer within [*] days after the date
on which the Antigen Evaluation Materials are received by the Non-Designating
Party (or within such longer negotiation period as the Parties may agree upon in
writing), if as of the date on which such period expires the Designating Party's
inclusion of such Target on its In-House Target List results in such Party [*],
the Designating Party shall remove such Antigen from its In-House Target List
and its License with respect to such Antigen shall automatically terminate,
unless as of such date such Party (y) exercises its rights under a Commercial
License with respect to such Antigen pursuant to Section 4.3 hereof, and (z)
selects such Target as a [*] Target pursuant to Section 5.3 hereof. In the event
that the Designating Party and the Non-Designating Party enter into a definitive
agreement as contemplated by this Section 5.1.3(d)(i) or if the Non-Designating
Party rejects or otherwise fails to respond to the Opt-In Offer within the [*]
day period (and any extensions thereof agreed to by the Parties pursuant to this
Section), the Designating Party shall have the right to retain the Target on its
In-House Target List and such Target shall [*], unless and until such Target is
removed from such Party's In-House Target List pursuant to clause (b) of Section
5.5 hereof.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-61-
(ii) Opt-In Process With Respect to Listed Targets.
In the event that the Designating Party desires to have the Non-Designating
Party collaborate on an In-House Project with respect to a Target that has
already been added by the Designating Party to its In-House Target List, the
Designating Party shall provide written notice to the other Party that it
desires to make an Opt-In Offer to the Non-Designating Party with respect to
such Target. Within [*] business days after the date of such notice, subject to
Section 5.1.3(d)(iii) hereof, the Designating Party shall provide to the
Non-Designating Party Antigen Evaluation Materials relating to such Target and
thereafter [*] to the Non-Designating Party in its review and analysis of such
materials. The Non-Designating Party shall have [*] days from the date on which
a complete set of Antigen Evaluation Materials are received to accept or reject
the Designating Party's Opt-In Offer; provided, however, that the
Non-Designating Party may request that the Designating Party consent to [*] days
within which to accept or reject the Opt-In Offer, [*]. In the event that the
Non-Designating Party accepts the Opt-In Offer, the Parties shall negotiate [*]
the terms and conditions of a definitive agreement; provided, however, that such
Target shall continue to [*] during the negotiation period. In the event that
the Non-Designating Party rejects the Opt-In Offer or that the Parties are not
otherwise able to reach agreement on the terms of a definitive agreement with
respect to the subject matter of the Opt-In Offer within [*] after the date on
which the Antigen Evaluation Materials are received by the Non-Designating Party
(or within such longer negotiation period as the Parties may agree upon in
writing), the Target shall continue to [*] unless and until such Target is
removed from such Party's In-House Target List pursuant to clause (b) of Section
5.5 hereof. In the event that the Designating Party and the Non-Designating
Party enter into a definitive agreement governing the subject matter of the
Opt-In Offer, the Target shall [*] as of the effective date of such definitive
agreement.
(iii) Antigen Evaluation Materials. With respect to
any Antigen Evaluation Materials to be provided by the Designating Party to the
Non-Designating Party pursuant to Section 5.1.3(d)(i) or 5.1.3(d)(ii) hereof,
the Designating Party shall provide to the Non-Designating Party any and all
Antigen Evaluation Materials that [*] select such Antigen as an In-House Target,
exercise rights with respect to such Antigen and/or make an Opt-In Offer with
respect to such Antigen. Subject to any applicable exclusion(s) set forth in
clauses (a) through (e) of Section 1.38 (Confidential Information), any and all
Antigen Evaluation Materials provided by the Designating Party to the
Non-Designating Party shall be deemed the Confidential Information of the
Designating Party and be subject to the terms and conditions of Article XIII
hereof. In the event that any Antigen Evaluation Materials to be provided
hereunder by the Designating Party include any material [*] as determined by the
Designating Party, the Designating Party shall notify the Non-Designating Party
and disclose to such Non-Designating Party the nature of such material and the
Parties shall confer [*]. In the event the Non-Designating Party determines that
the disclosure of such material is [*], the Designating Party shall have [*]. In
the event the Non-Designating Party determines [*], the Designating Party shall
have the right to [*] such materials to the Non-Designating Party or [*] the
Opt-In Offer in the event that [*].
(iv) [*] Relating to Opt-Ins. Each Party [*] to the
other Party that it shall exercise its rights under this Section 5.1.3(d) [*]
and [*].
5.2 [*] Target Selection.
5.2.1 [*] Target Rights. During the Term of the Agreement, each
Party shall accumulate rights to designate Targets on its In-House Target List
as [*] Targets (each a "[*] Target Right"), which rights shall accrue to each
Party on a Target-by-Target basis, commencing as of January 1, 2000, at an [*]
of [*] Targets in one year and [*] Targets in the next year, accruing at the
beginning of each Calendar Year, until, with respect to a Party, such Party has
accumulated [*] Target Rights in the aggregate (for exercise during the Term),
at which point [*] Target Rights shall accrue for such Party. A
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-62-
Party shall be permitted to select a Target on its In-House Target List as a [*]
Target only after a [*] Target Right has accrued to such Party. Once a [*]
Target Right is used by a Party such right shall be deemed to have expired (even
in the event that such Target is later redesignated by such Party as a [*]
Target or with respect to which Target such Party later implements an In-House
Project Status Change pursuant to Section 7.1 hereof) and, except in accordance
with the terms of Section 5.2.2 hereof, may [*]. A Target that is selected by a
Party as a [*] Target in accordance with Section 5.3 hereof may be redesignated
by such Party as a [*] Target, in which case the Designating Party shall be
required, pursuant to [*] to the other Party with respect to such Target [*]. By
way of example and without limitation, in the event that Kirin as the
Designating Party achieves [*] at the time when the Target is designated as a
[*] Target and [*] after the Target has been redesignated as a [*] Target, [*].
5.2.2 Substitution of [*] Targets.
(a) [*] Target Substitution. Subject to the terms of Section
5.2.2(b) hereof, in the event that the Designating Party, prior to the [*] with
respect to a [*] Target, (i) abandons research, development and/or
commercialization activities with respect to a [*] Target (and terminates its
Reservation License or Commercial License, as the case may be, with respect to
such Target), or (ii) redesignates the [*] Target as a [*] Target pursuant to
Section 5.3.1 hereof, such Party shall have the right to make a [*] Target
Substitution with respect to such [*] Target by providing prompt written notice
thereof to the other Party; provided, however, that [*] Party shall be permitted
to make [*] during the Term of this Agreement.
(b) [*] Target Substitution Fee. At the time that a Party makes
a [*] Target Substitution, such Party shall pay the other a [*] Target
Substitution Fee.
5.3 [*] Target Selection.
5.3.1 In General. Subject to the terms of Sections 5.1 and 5.3.2
hereof, each Party shall be permitted to select [*] In-House Targets as [*]
Targets during the Term of this Agreement. Upon selection by a Party of a Target
on its In-House Target List as a [*] Target, such Party shall [*]. A Target that
is selected by a Party as a [*] Target in accordance with Section 5.2 hereof may
be redesignated by such Party as a [*] Target, in which case the Designating
Party shall be required, pursuant to [*]. By way of example and without
limitation, in the event that Kirin as the Designating Party achieves [*] at the
time when the Target is designated as a [*] Target and [*] after the Target has
been redesignated as a [*] Target, Kirin shall [*].
5.3.2 Designation of [*] Targets in [*]. At any time on or after
the Effective Date, in the event that a Party desires to select an Antigen for
the exercise of rights under a License pursuant to Article IV hereof in
connection with an In-House Project and the exercise of such rights and the
corresponding designation of such Antigen on such Party's In-House Target List
would cause such Party to [*], such Party may [*] exercise such rights and
designate the Antigen as a Target on its In-House Target List, subject to the
availability of such Antigen as determined in accordance with Article III
hereof, by providing written notice thereof to the other Party and
simultaneously therewith (a) exercising its rights under a Commercial License
with respect to such Antigen pursuant to Section 4.3 hereof, (b) designating
such Antigen as a Target and noting the corresponding Designation Date on its
In-House Target List, and (c) selecting such Target as a [*] Target. Within [*]
days after the date of such notice, the Designating Party shall [*]. Such Target
shall remain on such Party's In-House Target List unless and until such Target
is removed pursuant to clause (b) of Section 5.5 hereof.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-63-
5.4 [*] Target Selection. A Party shall have the right in its sole
discretion to [*] a Target [*] its In-House Target List at any time during the
Term of this Agreement [*], in which event such Party shall promptly notify the
other Party thereof, whereafter such Target shall [*]. Thereafter, except to the
extent licensed under the Evaluation License granted to such Party in Sections
8.2.2 or 8.3.2 hereof, as the case may be, such Party shall have [*] with
respect to such Antigen; provided, however, that if such Party subsequently
determines that it or one of its Affiliates or In-House Collaborators desires to
pursue such Antigen, then, subject to receiving confirmation of availability
pursuant to Article III hereof, and the exercise of rights under a License with
respect to such Antigen pursuant to Article IV hereof, then subject to Section
5.1.2 hereof, such Party may [*] designate such Antigen as a Target and [*] it
to its In-House Target List.
5.5 Removing Targets from the [*] and the Target List. A Target that has
been designated by a Party as an In-House Target shall (a) [*] upon (i)
designation by such Party of such Target as a [*] Target, (ii) the occurrence of
the [*] by such Party (or any of its Affiliates or sublicensees) of a [*] Target
Product related to such Target in any Major Country, (iii) designation by such
Party of such Target as an [*] Target pursuant to Section 5.4 hereof, (iv) the
execution by the Parties of a separate written agreement pursuant to which the
Parties collaborate with respect to the development and commercialization of a
Collaboration Product against such Target, or (v) satisfaction of the applicable
conditions provided in Section 5.1.3(d)(i) hereof with respect to Opt-In Offers;
and (b) be removed by a Party from its In-House Target List if such Party
designates such Target as an [*] Target pursuant to Section 5.4 hereof or
terminates in its entirety the License to which such Target relates; provided,
however, that nothing contained in this Section 5.5 shall be interpreted to
alter or otherwise terminate any payment obligation, including, without
limitation, any royalty obligation applicable to products directed against such
Target pursuant to Section 10.6 hereof.
ARTICLE VI
PARTNER PROJECTS
6.1 Partner Projects. Each Party may conduct a Partner Project only in
accordance with the terms of this Article VI. Except as provided in Sections 6.2
[*] hereof, during the period beginning on December 27, 1999, and ending on the
last day of the Term, (a) Medarex represents that it (and Medarex Affiliates)
has entered and agrees that it (and its Affiliates) will enter into Project
Agreements governing one or more Medarex Partner Projects only with Third
Parties Headquartered in the Medarex Primary Promotional Area, and (b) except
for that certain Research and Commercialization Agreement effective as of April
15, 2001, entered into by Kirin with Corixa Corporation, Kirin represents that
it (and Kirin Affiliates) has entered and agrees that it (and its Affiliates)
will enter into Project Agreements governing one or more Kirin Partner Projects
only with Third Parties Headquartered in the Kirin Primary Promotional Area. In
addition, except as provided in Sections 6.2 and 6.3 hereof, during the period
beginning on December 27, 1999, and ending on the last day of the Term Medarex
represents that it (and its Affiliates) have entered and agrees that it (and its
Affiliates) will enter into Project Agreements governing one or more HuMAb
License Projects only with Third Parties Headquartered in the Medarex Primary
Promotional Area. The Parties shall discuss in good faith [*], whether the Kirin
Primary Promotional Area shall be expanded to include Third Parties
Headquartered in Australia and New Zealand, with a corresponding modification to
the Medarex Primary Promotional Area.
6.2 Partner Projects Involving Special Relationships. Notwithstanding the
terms of Section 6.1 hereof, if a Party (or any of its Affiliates) has a Special
Relationship with a Third Party Headquartered in the other Party's Primary
Promotional Area, and such Third Party wishes to enter into a Project Agreement
that governs one or more Partner Projects, or, in the case of Medarex, one or
more
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-64-
Partner Projects and/or HuMAb License Projects, with the Party (or any of its
Affiliates) with which it has the Special Relationship, then the Party (or its
Affiliate) with such Special Relationship may enter into such Project Agreement
with such Third Party, subject to the written consent of the other Party, which
consent shall not be unreasonably withheld. For purposes of clarification, (a)
"consent" for purposes of this Section 6.2 shall mean consent by a Party as to
the other Party's entering into such Project Agreement governing the Partner
Project(s) and/or HuMAb License Project(s), as the case may be, rather than
consent as to the form or content of such proposed Project Agreement, and (b)
the fact that a Party consents to the other Party's entering into any such
Project Agreement with a Third Party shall not make it unreasonable for the
Party granting such consent to later withhold its consent to such other Party's
entering into a subsequent Project Agreement that governs one or more Partner
Project(s) and/or HuMAb License Project(s), as the case may be, with the same
Third Party.
6.3 [*] Advertisements or Other Promotions.
6.3.1 In General. Subject to the terms and conditions of Section
6.3.2 hereof, each of Medarex (and its Affiliates) and Kirin (and its
Affiliates) may advertise and/or promote the Medarex Mice, Kirin Mice, and
KM-Mice, including, without limitation, publishing papers regarding any such
technology and presenting any such technology at conferences, anywhere in the
world. [*], if [*] advertisement or promotion undertaken by a Party (or any of
its Affiliates), such Party (or any of its Affiliates) is [*] by a Third Party
Headquartered in the [*] Primary Promotional Area regarding the possibility of
entering into a Project Agreement that governs one or more Partner Projects, or,
in the case of Medarex, one or more Partner Projects and/or HuMAb License
Projects, such Party (or its Affiliate) shall [*] such Third Party that the [*]
has [*] with respect to [*] governing such [*], and, in the case of [*] and/or
[*], in that [*]; provided, however, that if such [*] with the Party (or its
Affiliate) that it [*] and [*], then such [*] may [*] such [*] governing such
[*], and, in the case of [*], such [*] and/or [*], with [*], subject to the [*]
of the [*], which [*]. For purposes of clarification, (a) [*] for purposes of
this Section 6.3.1 shall mean [*] by a Party as to the [*] such [*] governing
the [*] and/or [*], as the case may be, [*] as to the [*] of such [*], and (b)
the fact that a Party [*] to the [*] any such [*] with a [*] shall [*] for the
Party [*] to [*] to such [*] a [*] that governs one or more [*] and/or [*], as
the case may be, with the [*].
6.3.2 Consultation With Respect to Advertising and Promotion. In
the event that Medarex (or any of its Affiliates) engages in advertising and/or
promotion with respect to the Kirin Mice or the KM-Mice, or Kirin (or any of its
Affiliates) engages in advertising or promotion with respect to the Medarex Mice
or the KM-Mice, and the other Party objects to such Party's (or its Affiliates)
advertising or promotion, such other Party may provide notice thereof to the
Party engaging in such advertising or promotion (or whose Affiliate is engaging
in such advertising or promotion) and the Parties shall promptly consult in good
faith to address and resolve any such reasonable objections relating to such
advertising or promotion.
6.4 Partner Opportunities. The Requesting Party may request that the
Non-Requesting Party notify one or more of the Non-Requesting Party's Partners
that the Requesting Party desires to negotiate with such Partner(s) for
Commercialization Rights in the Requesting Party's Primary Promotional Area with
respect to Antibody Products directed against Targets to which such Partner(s)
has rights under this Agreement. Upon receipt of any notice identifying any such
Partner, the Non-Requesting Party shall promptly provide such notice to such
Partner and shall provide [*] to the Requesting Party in facilitating the
initiation of the negotiation of any such Commercialization Rights between such
Partner and the Requesting Party.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-65-
6.5 Covenants.
6.5.1 Terms and Conditions of Project Agreements.
(a) Third Party Agreements and Grant of Access to Technology.
Except as provided in Section 6.5.1(b) hereof, Medarex covenants to Kirin and
Kirin covenants to Medarex that during the Term of this Agreement neither it nor
its Affiliates will enter into a Project Agreement unless the terms and
conditions of the Project Agreement:
(i) permit such Party to notify the other Party of
the existence of such agreement and the identity of such Third Party, within [*]
days after the effective date of such agreement, together with the identity of
the Collaboration Mice that are the subject of the License or sublicense; and
(ii) in the case of a Project Agreement governing a
Partner Project and/or In-House Project, permit such Party to notify the other
Party, in the event of any transfer of Collaboration Mice and/or Antibodies or
Antibody Materials produced therefrom to such Third Party in connection with
such project(s) within [*] days after such transfer, (A) if by Medarex the fact
that Kirin Mice or KM-Mice were transferred to such Third Party and/or the fact
that Antibodies or Antibody Materials were produced using the Kirin Mice or
KM-Mice and such Antibodies and/or Antibody Materials were transferred to such
Third Party, or, (B) if by Kirin the fact that Medarex Mice or KM-Mice were
transferred to such Third Party and/or the fact that Antibodies or Antibody
Materials were produced using the Medarex Mice or KM-Mice were transferred to
such Third Party.
(b) Antigen Disclosures. Except as provided in this Section
6.5.1(b), Medarex covenants to Kirin and Kirin covenants to Medarex that during
the Term neither it nor any of its Affiliates will enter into a Project
Agreement governing a Partner Project, which Project Agreement provides for the
grant by such Party to a Partner of a sublicense under a Reservation License or
a Commercial License with respect to Antibodies raised against and with affinity
for a particular Antigen, and/or Antibody Products containing such Antibodies,
in each case in connection with such Partner Project, unless the terms and
conditions of such Project Agreement permit such Party to notify the other Party
of the identity of any such Antigen and the identity of the Partner within [*]
days after the earlier of (i) the grant by the contracting Party to the Partner
of a sublicense under a Commercial License with respect to such Antigen in
connection with such Partner Project, or (ii) public disclosure of the fact that
a particular Antibody (or Antibody Product) was developed by the Partner in
connection with such Partner Project through use of the non-contracting Party's
mice or Technology and/or the KM-Mice or KM Patent Rights, as the case may be.
For the avoidance of doubt, if a Third Party will not agree to permit a Party to
include in the Project Agreement, terms that satisfy the requirements of
Sections 6.5.1(a) and 6.5.1(b), such Party (or its Affiliate) may not enter into
the Project Agreement with such Third Party governing a Partner Project;
provided, however, that in the case of [*] (or a [*] Affiliate, as permitted
under Section 8.5.3 to the extent applicable) as the contracting Party, [*] or
its Affiliate [*] a [*] governing a [*] or [*] with a Third Party which [*]
after [*] to have [*], but [*], in that or any other agreement or legally
binding understanding, [*] such Third Party [*] or other [*] the [*] unless the
Third Party [*].
Notwithstanding the foregoing, in the event that Medarex, after [*], is [*] the
agreement of a Third Party to [*] such [*] to [*] in accordance with this
Section 6.5.1(b), it shall not be deemed a [*] to [*] to [*] such [*] and Kirin
shall [*] to [*] or otherwise bring [*] for [*] to [*] the Third Party the [*]
such [*] or for [*] a [*] governing a [*] or [*] with the Third Party [*] such
[*] to the [*] of such [*]; provided,
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-66-
however, that nothing herein is intended to limit [*], if any, or [*], if any,
with respect to the [*] of any other [*] or [*] in this Agreement, including
without limitation, [*] of a [*] or other [*] the [*] to a Third Party which
[*]. For the avoidance of doubt, for purposes of this Section 6.5.1(b), Medarex
shall [*] to [*] the [*] in the [*] of such [*]."
6.5.2 Project Agreement Disclosure Requirements.
(a) Third Party Agreements and Grant of Access to Technology.
Except as provided in Section 6.5.2(b), Medarex covenants to Kirin and Kirin
covenants to Medarex that during the Term it shall provide written notice to the
other Party with respect to Project Agreements entered into during the Term as
follows:
(i) the existence of such agreement and identity of such
Third Party, within thirty (30) days after the effective date of such agreement,
together with the identity of the Collaboration Mice that are the subject of the
License or sublicense; and
(ii) in the case of a Project Agreement governing a
Partner Project and/or In-House Project, in the event of any transfer of
Collaboration Mice and/or Antibodies or Antibody Materials produced therefrom to
such Third Party in connection with such project(s) within [*] days after such
transfer, (A) if by Medarex the fact that Kirin Mice or KM-Mice were transferred
to such Third Party and/or the fact that Antibodies or Antibody Materials were
produced using the Kirin Mice or KM-Mice and such Antibodies and/or Antibody
Materials were transferred to such Third Party, or, (B) if by Kirin the identity
of Medarex Mice or KM-Mice and/or the fact that Antibodies or Antibody Materials
as were produced using the Medarex Mice or KM-Mice and transferred to such Third
Party.
(b) Antigen Disclosures. During the Term of this Agreement, if a
Party desires to know the [*] of an [*] for which a particular [*] has [*] a [*]
a [*] or a [*] with respect to [*], and/or [*], in each case in connection with
such Partner Project, such Party shall provide written notice thereof to the
other Party within [*] days after the receipt of such notice from the other
Party disclosing the identity of such Partner pursuant to Section 6.5.2(a)
hereof. From and after the receipt of such notice from the non-contracting
Party, the contracting Party shall notify the non-contracting Party of the
identity of any such Antigen and the identity of such Partner within [*] days
after the earlier of (i) the grant by the contracting Party to the Partner of a
sublicense under a Commercial License with respect to such Antigen in connection
with such Partner Project, or (ii) public disclosure of the fact that a
particular Antibody (or Antibody Product) was developed by the Partner in
connection with such Partner Project through use of the non-contracting Party's
mice or Technology and/or KM-Mice or KM Patent Rights, as the case may be.
Notwithstanding the foregoing, in the event that Medarex, [*] is [*] the
agreement of a Third Party [*] in accordance with this Section 6.5.2(b), it
shall not be deemed a [*] to [*] to [*] such [*] and Kirin shall [*] or
otherwise bring [*] for [*] or [*] to [*] the Third Party the [*] such [*] or
for [*] a [*] governing a [*] or [*] with the Third Party [*] such [*] to the
[*] of such [*]; provided, however, that nothing herein is intended to limit
[*], if any, or [*], if any, with respect to the [*] of any other [*] or [*] in
this Agreement, including without limitation, [*] a [*] or other [*] the [*] to
a Third Party which [*]. For the avoidance of doubt, for purposes of this
Section 6.5.2(b), [*] shall [*] to [*] to the Third Party in the [*] of such [*]
6.5.3 [*] The disclosure of information pursuant to Section 6.5.2
hereof shall be subject to the terms and conditions of [*] hereof. Each Party
represents, warrants, and covenants to the other Party that it will [*]
information provided to it by the other Party pursuant to Section 6.5.2 hereof,
and will [*] such information.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-67-
6.5.4 [*] regarding HuMAb Collaboration Project. Medarex shall not
enter into, or amend or modify, an agreement with one or more Medarex Affiliates
or Third Parties such that such agreement would be deemed to provide for the
conduct of a HuMAb Collaboration Project if Medarex's [*]. In the event that
Medarex enters into, amends, or modifies such an agreement in breach of the [*]
contained in this Section 6.5.4, such project shall be deemed a [*] for purposes
of this Agreement.
6.6 [*] of Collaboration Mice to Partners. Medarex agrees that, from and
after the Effective Date, at the time it offers to a Partner or prospective
Partner an opportunity to obtain a license (or a sublicense) to use the KM-Mice
and/or Medarex Mice, Medarex shall [*] such Person that such Person may [*] from
[*] a [*] (or a [*]) to [*] the [*] (provided that [*] shall be obligated to so
[*] such Person with respect to [*]) and, if [*] the [*] were [*], the [*] and
[*], in each case on terms that are [*] the terms on which [*] to such Person
the [*] to [*] the [*] (or [*]) to [*] the [*]. Kirin agrees that, from and
after the Effective Date, at the time it offers to a Partner or prospective
Partner an opportunity to obtain a license (or a sublicense) to use the KM-Mice,
TC Mice and/or HAC Mice, Kirin shall [*] such [*] that such [*] may also [*]
from [*] a [*] (or a [*]) to [*] the [*] and, if [*] were [*], the [*] and [*],
in each case on terms that are [*] the terms on which [*] to such Person the [*]
to [*] the [*] (or [*]) to [*] the [*], as the case may be. Notwithstanding the
foregoing, a Party may relieve the other Party of such other Party's affirmative
obligations pursuant to this Section 6.6 [*] by delivering to such other Party
written notice thereof (which notice may be revoked by subsequent written notice
from the original notifying Party to the other Party reinstating such
obligation(s) pursuant to this Section 6.6 effective no earlier than [*] days
following the delivery of such notice).
ARTICLE VII
CHANGE IN STATUS OF IN-HOUSE PROJECT OR PARTNER PROJECT
7.1 Change in Rights Related to Target Designated for In-House Project.
7.1.1 In General. If at any time after a Party has designated a
Target on its In-House Target List in connection with an In-House Project, the
Party designating the Target (or the Affiliate of such Party, in the case of
In-House Projects involving such Party's Affiliate) desires to effect an
In-House Project Status Change, such Party (or its Affiliate, as the case may
be) shall be permitted to effect such change only if such Party complies with
this Section 7.1 and provides to the other Party (a) prior written notice of
such proposed change; and (b) prompt written notice following implementation of
such change, indicating the In-House Project Status Change Date.
7.1.2 Requirements for In-House Project Status Change. In the event
that a Party has designated a Target (in accordance with Article V hereof) as an
In-House Target in connection with a project and the Party and/or such Party's
Affiliate Opts Out with respect to such Target [*], from and after the In-House
Project Status Change Date, the project shall nevertheless continue to be an
In-House Project such that (a) the Primary Promotional Area restrictions set
forth in Section 6.1 hereof shall not apply, and (b) such Target shall continue
to [*] unless and until one of the conditions in clause (a) of Section 5.5
hereof has been satisfied with respect to such Target.
7.1.3 Designation of Targets In Connection With In-House Project
Status Change. For purposes of Section 7.1.2, if prior to the In-House Project
Status Change Date the Party seeking to implement such change has not yet
designated the Target as a [*] Target or a [*] Target pursuant to Article V
hereof, such Party shall designate the Target as either a [*] Target or a [*]
Target in the notice provided to the other Party pursuant to Section 7.1.1
hereof, and such designation (i.e., as either a [*] Target or a [*] Target)
shall continue to apply with respect to the Designating Party's obligations
under
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-68-
[*] hereof. If prior to the In-House Project Status Change Date with respect to
such project, the Party seeking to implement such change has designated the
Target as a [*] Target or a [*] Target pursuant to Article V hereof, such
designation shall continue to apply with respect to the Designating Party's
obligations under [*] hereof, unless and until, with respect to a Target
designated as a [*] Target, such Designating Party redesignates such [*] Target
as a [*] Target pursuant to Section 5.3.1 hereof.
7.1.4 Covenant Regarding Change in Nature of In-House Project
Rights. Neither Party shall initially enter into a Project Agreement with
respect to an In-House Project and later effect (or attempt to effect) an
In-House Project Status Change with respect to such In-House Project pursuant to
Section 7.1.2 hereof, if such Party's [*] in structuring such project as an
In-House Project and later effecting (or attempting to effect) an In-House
Project Status Change was [*] set forth in Section [*] hereof or such Party's
[*].
7.2 Change in Rights Related to Target Selected for Partner Project.
7.2.1 In General. If at any time after a Party who has exercised its
rights under a License with respect to a particular Target desires to effect a
Partner Project Status Change to convert the project to an In-House Project,
such Party shall be permitted to effect such change and convert the project to
an In-House Project only if such Party has not, and will not as a result of
effecting such change, [*] for In-House Projects. In the event that such change
would result in such Party [*], such Party may not effect such change in the
nature of its rights unless such Party designates such Target as a [*] Target
pursuant to Section 7.2.2 hereof and within [*] days after the Partner Project
Status Change Date [*].
7.2.2 Requirements for Conversion. If a Party is permitted to
convert a Partner Project to an In-House Project pursuant to Section 7.2.1
hereof, it may do so only if it provides to the other Party (a) prior written
notice of such proposed change; and (b) prompt written notice following
implementation of such change, indicating the Partner Project Status Change
Date. As part of such notice, the notifying Party shall designate the Target as
either a [*] Target pursuant to Section 5.2 hereof or a [*] Target pursuant to
Section 5.3 hereof. From and after the Partner Project Status Change Date, the
project shall be treated as an In-House Project for purposes of this Agreement,
including, without limitation, the terms and conditions of [*] hereof; provided,
however, that in the event that the Designating Party designates the Target as a
[*] Target, it shall be required to [*] the Partner Project Status Change Date.
If one or more [*] has already occurred with respect to such Target as of the
Partner Project Status Change Date, [*]. Upon the conversion of a Partner
Project to an In-House Project pursuant to this Section 7.2, the Party making
such conversion shall [*] thereunder.
ARTICLE VIII
GRANTS OF LICENSES
8.1 Present Grants of Licenses. The intent and effect of the grant of each
License in this Article VIII is to create a present license grant and right in
favor of the licensee Party as to such License, subject to the terms and
conditions of this Agreement. Subject to any contrary and nonwaivable
requirements of applicable law, the Parties intend for the license grants herein
to be construed in a manner which preserves (to the maximum extent) for the
licensee Parties each of the benefits of the bargain set forth in this
Agreement, rather than in a manner that may leave the provision voidable,
rejectable or otherwise terminable or unenforceable by operation of applicable
law. Without limitation of the foregoing, the Parties intend that the rights of
a non-debtor Party should survive to the maximum extent permitted by applicable
law, notwithstanding a rejection of this Agreement by a debtor Party pursuant to
Section 365 of the Bankruptcy Code or pursuant to the Japanese bankruptcy law,
as applicable.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-69-
8.2 Medarex Licenses to Kirin.
8.2.1 Breeding License. Subject to Sections 8.2.5 and 8.11 hereof and
the other terms and conditions of this Agreement, Medarex hereby grants to
Kirin, and Kirin hereby accepts, a non-exclusive, non-transferable (except
pursuant to Section 18.4 hereof), fully paid up, royalty-free, worldwide,
perpetual (subject to any rights of termination provided in this Agreement)
right and license, with rights to sublicense as permitted under Section 8.5.1(a)
hereof, under the Medarex Technology and Medarex's interest in the KM Patent
Rights and KM Know-How, and any Medarex Improvements to any of the foregoing, to
research, develop, breed or otherwise make or manipulate, use, import, and
export (but not to sell, offer to sell, lease, offer to lease, or otherwise
transfer title to) Collaboration Mice at any and all of Kirin's (or any Kirin
Affiliate's) facilities anywhere in the world, including, without limitation,
subject to Section 8.5.1(a) hereof, the facilities of a Third Party contract
service provider to which Kirin contracts out the maintenance, propagation
and/or manipulation of Collaboration Mice in connection with a Services Project.
Kirin acknowledges that Article IX hereof governs the availability and transfer
of Medarex Materials that may, but need not, be used by Kirin and its
sublicensees in the enjoyment of the right and license granted by Medarex to
Kirin in this Section 8.2.1.
8.2.2 Evaluation License. Subject to Sections 8.2.5 and 8.11 hereof
and the other terms and conditions of this Agreement, Medarex hereby grants to
Kirin, and Kirin hereby accepts, a non-exclusive, non-transferable (except
pursuant to Section 18.4 hereof), fully paid up, royalty-free, worldwide,
perpetual (subject to any rights of termination provided in this Agreement),
right and license, with rights to sublicense as permitted under Section 8.5.1(b)
hereof, under the Medarex Technology and Medarex's interest in the KM Patent
Rights and KM Know-How, and any Medarex Improvements to any of the foregoing, to
immunize Collaboration Mice to raise Antibodies (and Antibody Materials related
thereto) against, and with affinity for, Antigens, and to use Mice Materials
derived from any Collaboration Mice to generate Antibodies and/or to evaluate
such Antibodies, in each case solely for research purposes (but not for clinical
development), and to determine whether Kirin desires to exercise its rights
under a Reservation License or Commercial License with respect to any such
Antibodies, Antibody Materials related thereto, or Antibody Products containing
such Antibodies, and/or whether any of Kirin's Affiliates, Partners or In-House
Collaborators desires to acquire a sublicense under any such License.
8.2.3 Reservation Licenses. On a Kirin Reservation Target by Kirin
Reservation Target basis, with respect to each Kirin Reservation Target, subject
to Sections 8.2.5 and 8.11 hereof and the other the terms and conditions of this
Agreement, Medarex hereby grants to Kirin, and Kirin hereby accepts, a
non-exclusive, non-transferable (except pursuant to Section 18.4 hereof),
royalty-free, worldwide right and license during the Reservation License Period
for such Kirin Reservation Target, with rights to sublicense as permitted under
Section 8.5.1(c) hereof, under the Medarex Technology and Medarex's interest in
the KM Patent Rights and KM Know-How, and any Medarex Improvements to any of the
foregoing, to immunize Collaboration Mice to raise Antibodies (and Antibody
Materials related thereto) against, and with affinity for, such Kirin
Reservation Target, and to use Mice Materials derived from any Collaboration
Mice to generate Antibodies and/or to evaluate such Antibodies, in each case
solely for research purposes (but not for clinical development), and to
determine whether Kirin desires to exercise its rights under a Commercial
License with respect to such Kirin Reservation Target pursuant to Section 4.3
hereof and/or whether any of Kirin's Affiliates, Partners, or In-House
Collaborators desires to acquire a sublicense under any such Commercial License.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-70-
8.2.4 Commercial Licenses.
(a) Antigen Exclusive Commercial Licenses. On a Selected
Antibody-by-Selected Antibody basis, with respect to [*] with respect to each
Kirin Exclusive Commercial Target pursuant to Sections 4.4.1(b) and 4.4.2
hereof, subject to Sections 8.2.5, 8.2.7(b), 8.2.7(c), 8.2.7(d), 8.2.8(b),
8.2.8(c), and 8.11 hereof and the other terms and conditions of this Agreement,
Medarex hereby grants to Kirin, and Kirin hereby accepts, a worldwide, exclusive
(even as to Medarex), non-transferable (except pursuant to Section 18.4 hereof),
royalty-bearing, perpetual (subject to any rights of termination provided in
this Agreement), right and license, with rights to sublicense as permitted under
Section 8.5.1(d) hereof, under the Medarex Technology and Medarex's interest in
the KM Patent Rights and KM Know-How, and any Medarex Improvements to any of the
foregoing, to Exploit and/or have Exploited in the Field (i) such Selected
Antibody (and Antibody Material related thereto), and/or (ii) Collaboration
Products containing such Selected Antibody(ies) (and Antibody Materials related
thereto). Medarex represents, warrants and covenants to Kirin that, except as
provided in Section 8.2.7 or 8.2.8 hereof, Kirin shall have rights of
exclusivity as set forth in the preceding sentence and none of Medarex or its
assignees or successors shall grant or shall have granted to any other Person
any license or other right to Exploit and/or have Exploited (which in the case
of past grants shall refer to a license or other right that remains in effect),
(x) Antibodies (and Antibody Materials related thereto) raised against and with
affinity for the Kirin Exclusive Commercial Target against which such Selected
Antibody(ies) was raised, or (y) Antibody Products containing Antibodies (and
Antibody Materials related thereto) raised against and with affinity for such
Kirin Exclusive Commercial Target.
(b) Antigen Non-Exclusive Commercial Licenses. On a Selected
Antibody-by-Selected Antibody basis, with respect to [*] with respect to each
Kirin Non-Exclusive Commercial Target pursuant to Sections 4.4.1(a) and 4.4.2
hereof, subject to Sections 8.2.5, 8.2.7(b), 8.2.7(c), 8.2.7(d), 8.2.8(b),
8.2.8(c), and 8.11 hereof and the other terms and conditions of this Agreement,
Medarex hereby grants to Kirin, and Kirin hereby accepts, a worldwide, exclusive
(even as to Medarex), non-transferable (except pursuant to Section 18.4 hereof),
royalty-bearing, perpetual (subject to any rights of termination provided in
this Agreement), right and license, with rights to sublicense as permitted under
Section 8.5.1(d) hereof, under the Medarex Technology and Medarex's interest in
the KM Patent Rights and KM Know-How, and any Medarex Improvements to any of the
foregoing, to Exploit and/or have Exploited in the Field (i) such Selected
Antibody (and Antibody Material related thereto), and/or (ii) Collaboration
Products containing such Selected Antibody(ies) (and Antibody Materials related
thereto). For the avoidance of doubt, nothing contained in this Section 8.2.4(b)
shall prohibit Medarex from granting to any other Person a license to Exploit
and/or have Exploited Antibodies (and Antibody Materials related thereto), other
than such Selected Antibody(ies), raised against and with affinity for such
Kirin Non-Exclusive Commercial Target.
(c) Antigen Semi-Exclusive Commercial License. On a Selected
Antibody-by-Selected Antibody basis, with respect to [*] with respect to each
Kirin Semi-Exclusive Commercial Target pursuant to Sections 4.4.1(b) and 4.4.2
hereof, subject to Sections 8.2.5, 8.2.7(a), 8.2.7(b), 8.2.7(c), 8.2.7(d),
8.2.8(a), 8.2.8(b), 8.2.8(c), and 8.11 hereof and the other terms and conditions
of this Agreement, Medarex hereby grants to Kirin, and Kirin hereby accepts, a
worldwide, exclusive (subject specifically to Sections 8.2.7(a) and 8.2.8(a))
(even as to Medarex), non-transferable (except pursuant to Section 18.4 hereof),
royalty-bearing, perpetual (subject to any rights of termination provided in
this Agreement), right and license, with rights to sublicense as permitted under
Section 8.5.1(d) hereof, under the Medarex Technology and Medarex's interest in
the KM Patent Rights and KM Know-How, and any Medarex Improvements to any of the
foregoing, to Exploit and/or have Exploited in the Field (i) such Selected
Antibody (and Antibody Material related thereto), and/or (ii) Collaboration
Products containing such Selected Antibody(ies) (and Antibody Materials related
thereto). Medarex represents, warrants and
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-71-
covenants to Kirin that, except as provided in Section 8.2.7 or 8.2.8 hereof,
Kirin shall have rights of exclusivity as set forth in the preceding sentence
and none of Medarex, its assignees, or successors shall grant or shall have
granted to any other Person any license or other right to Exploit and/or have
Exploited (which in the case of past grants shall refer to a license or other
right that remains in effect), (x) Antibodies (and Antibody Materials related
thereto) raised against and with affinity for the Kirin Semi-Exclusive
Commercial Target against which such Selected Antibody(ies) was raised, or (y)
Antibody Products containing Antibodies (and Antibody Materials related thereto)
raised against and with affinity for such Kirin Semi-Exclusive Commercial
Target.
8.2.5 Sublicenses Under Medarex In-License Agreements.
(a) In General. Subject to Section 8.11 hereof and the other
terms and conditions of this Agreement, including this Section 8.2.5, with
respect to any Medarex Technology that Medarex Controls pursuant to licenses
granted to Medarex by Third Parties in any Medarex In-License Agreements, the
licenses granted by Medarex to Kirin in Sections 8.2.1, 8.2.2, 8.2.3 and 8.2.4
hereof shall include sublicenses under such Medarex Technology (with the rights
for Kirin to grant further sublicenses only as permitted in Sections 8.5.1
hereof) (i) for the same scope and field of use, with the same degree of
exclusivity (i.e., non-exclusive, exclusive, or semi-exclusive), and with
respect to the same Selected Antibodies, Antibody Materials and Collaboration
Products as specified in the relevant license grant in Sections 8.2.1, 8.2.2,
8.2.3 and 8.2.4, as applicable, (ii) subject to the same pre-existing grants and
retained rights as apply to the corresponding grants in Sections 8.2.1, 8.2.2,
8.2.3 and 8.2.4 hereof, as applicable, and (iii) on the terms and conditions
that apply under the applicable Medarex In-License Agreement to the exercise by
any of Medarex's sublicensee(s) of such sublicense(s) (excluding any
payment-related terms thereof unless otherwise expressly provided in Article X
hereof), provided, however, that clause (iii) hereof shall in no way affect the
warranty made by Medarex to Kirin under Section 8.5.1(d)(ii).
(b) DNX Agreement. The sublicense granted by Medarex to Kirin
under the DNX Agreement in Section 8.2.5(a) hereof shall include a sublicense
for the right to (i) [*]; provided, however, that the sublicense granted to
Kirin by Medarex under the DNX Agreement shall [*]. The terms [*] shall have the
meanings set forth on Exhibit B hereto.
(c) MRC Agreement. The sublicense granted by Medarex to Kirin
under the MRC Agreement in Section 8.2.5(a) hereof shall include a sublicense
under all of the license rights granted to Medarex in [*] of the MRC Agreement,
except that Medarex [*] (as that term is defined in the MRC Agreement) [*].
(d) Notice to Medarex. In the event that Kirin grants to an
Affiliate, Partner or In-House Collaborator, pursuant to Article VIII hereof and
the other terms and conditions of this Agreement, any sublicense or other rights
under any Medarex In-License Agreement(s), Kirin shall provide to Medarex
written notice thereof within [*] business days of such grant by Kirin, which
notice shall identify such Person and the Medarex In-License Agreement(s) with
respect to which such sublicense or other rights were granted by Kirin.
8.2.6 Trademark License Granted by Medarex to Kirin.
(a) Grant. Subject to Section 8.11 hereof and the other terms
and conditions of this Agreement, Medarex hereby grants to Kirin a limited,
worldwide, non-exclusive, non-assignable, royalty-free, right and license during
the Term, with rights to sublicense as permitted under Section 8.5.1(f), to use
the Medarex Trademarks and the KM Trademarks in connection with the
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-72-
Exploitation of the Medarex Mice, Medarex Technology, KM-Mice, KM Patent Rights,
KM Know-How, Collaboration Antibodies, and/or Collaboration Products in
accordance with the terms and conditions of this Agreement, in accordance with
Medarex's corporate identity and trademark use guidelines as may be provided in
writing by Medarex to Kirin from time to time, and (i) as required by applicable
law; (ii) as reasonably necessary for the exercise of rights granted to Kirin
pursuant to Section 6.3.1 hereof; or (iii) otherwise as agreed in writing by
Medarex, such agreement not to be unreasonably withheld.
(b) Quality Control. Subject to Section 8.11 hereof, Kirin,
upon written request of Medarex, shall promptly furnish Medarex with
representative samples showing use of the Licensed Trademarks by Kirin (and any
of its Affiliates and sublicensees) and, upon written notification from Medarex,
shall cease, or modify in accordance with Medarex's reasonable instructions (or
cause Kirin Affiliates and sublicensees to cease, or modify in accordance with
Medarex's reasonable instructions), any use of the Licensed Trademarks that
Medarex reasonably deems not to be in compliance with its permitted and
reasonable standards and any applicable laws and regulations.
(c) Covenants Relating to Use of Trademarks. Kirin represents,
warrants and covenants that, it will not (i) use any Medarex Trademarks or any
part thereof as part of Kirin's corporate name; (ii) use any new Trademark
confusingly similar to the Medarex Trademarks; or (iii) hereafter register in
any country any Trademark resembling or confusingly similar to the Medarex
Trademarks.
8.2.7 Licenses Subject to Pre-Existing Grants.
(a) Each Kirin Antigen Semi-Exclusive Commercial License
granted by Medarex to Kirin pursuant to Section 8.2.4(c) hereof with respect to
a particular Kirin Semi-Exclusive Commercial Target shall be subject to any
existing grant of licenses, options, or rights of refusal or negotiation, in
each case granted by Medarex prior to the Effective Date to a Third Party under
the Medarex Technology and Medarex's interests in the KM Patent Rights or KM
Know-How with respect to one or more Antibodies (which Antibodies in the case of
an existing grant of license shall be listed on the Antibody Sequence List
pursuant to Article II hereof) against the Antigen with respect to which rights
are granted in the Kirin Antigen Semi-Exclusive Commercial License.
(b) Each Commercial License granted by Medarex to Kirin
pursuant to Section 8.2.4 hereof shall be subject to any exercise of rights by
Medarex prior to the Effective Date, and any existing grant of licenses,
options, or rights of refusal or negotiation, in each case granted by Medarex
prior to the Effective Date to Kirin or a Third Party under the Medarex
Technology and Medarex's interests in the KM Patent Rights and/or KM Know-How to
Exploit Antibodies (and Antibody Materials related thereto) with affinity for
the Antigen that is the subject of the Commercial License but raised against
another Antigen (which Antigen in the case of an existing license shall be
listed on an Antigen List pursuant to Article II hereof).
(c) Each Commercial License granted by Medarex to Kirin
pursuant to Section 8.2.4 hereof shall be subject to any existing grant of
licenses, options, or rights of refusal or negotiation, in each case granted by
Medarex prior to the Effective Date to Kirin or a Third Party under the Medarex
Technology and Medarex's interests in the KM Patent Rights and/or KM Know-How to
perform research and to determine whether such licensee desires to acquire a
license under such technology with respect to Antibodies (and Antibody Materials
related thereto) raised against and with affinity for Antigens and/or Antibody
Products containing Antibodies (and Antibody Materials related thereto).
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-73-
(d) The Parties acknowledge and agree that pursuant to the
Cross License Agreement, a copy of which has been provided to Kirin, Medarex has
granted a worldwide, non-exclusive license under the Patent Rights (as the term
is defined and used under the Cross License Agreement) to use for research, and
to develop, make, have made, use, import, export or otherwise transfer physical
possession of, sell, lease, offer to sell or lease, or otherwise transfer title
to Antibody Products (as defined in the Cross License Agreement).
(e) Notwithstanding anything else contained in this Agreement,
in the event that Medarex grants to Kirin a license (or a sublicense) in Section
8.2 hereof or Medarex grants to a Kirin Affiliate, Kirin Partner or Kirin
In-House Collaborator a license (and/or a sublicense) in a Direct Sublicense
Agreement, which license (and/or sublicense) is [*], Medarex shall [*] in the
Field under such Technology and/or Medarex's interest in the KM Patent Rights
and/or KM Know-How, as the case may be, with respect to the relevant
antibody(ies) in the relevant country(ies). Pursuant to Section 14.4 hereof [*]
with respect thereto whether or not [*], and pursuant to Section [*] hereof,
Medarex shall not [*]. This Section 8.2.7(e) shall not apply to grants of [*] by
Medarex from and after the Effective Date of its obligations to (a) follow the
procedures set forth in Section 2.3 hereof with respect to (i) the listing of
Antigens on any Antigen Lists that Medarex has an obligation to maintain
pursuant to Section 2.1.1 hereof, or (ii) the listing of Antibodies on the
Antibody Sequence List; or (b) respond to (i) Antigen Availability Inquiries
pursuant to Section 3.3 hereof, or (ii) Antibody Availability Inquiries pursuant
to Section 3.5 hereof, with respect to which [*].
8.2.8 Retained Rights.
(a) Notwithstanding anything in Section 8.2 of this Agreement
to the contrary, in the case of each Kirin Antigen Semi-Exclusive Commercial
License granted by Medarex to Kirin pursuant to Section 8.2.4(c) hereof, Medarex
hereby retains the right to (i) grant licenses, options, or rights of refusal or
negotiation under the Medarex Technology and Medarex's interests in the KM
Patent Rights and KM Know-How to Medarex's Affiliates, Kirin, and Third Parties,
to Exploit one or more Antibodies (and/or Antibody Products containing such
Antibodies) raised against and with affinity for the Antigen that is the subject
of such Antigen Semi-Exclusive Commercial License in effect pursuant to any
agreement entered into prior to the date on which the Antigen Semi-Exclusive
Commercial License is first exercised in accordance with the terms and
conditions of Article IV hereof, and (ii) exercise its rights under its
Technology to Exploit one or more Antibodies (and/or Antibody Products
containing such Antibodies) raised against and with affinity for the Antigen
that is the subject of such Antigen Semi-Exclusive Commercial License, in each
case, in connection with an active research, development and/or
commercialization project of Medarex, which Medarex can demonstrate, based on
its written records and the information contained on the Medarex Non-Exclusive
Antigen List, was commenced consistent with the terms of this Agreement before
the date on which such Antigen Semi-Exclusive Commercial License is exercised in
accordance with the terms and conditions of Article IV hereof; provided,
however, that Medarex does not retain the rights provided in clause (i) or (ii)
of this Section 8.2.8(a) with respect to any [*] for such License.
(b) Notwithstanding anything in Section 8.2 of this Agreement
to the contrary, in the case of each Commercial License granted by Medarex to
Kirin pursuant to Section 8.2.4 hereof, Medarex hereby retains the right to
Exploit, and to grant licenses, options, or rights of refusal or negotiation
under the Medarex Technology and its interest in the KM Patent Rights and KM
Know-How to Medarex Affiliates, Kirin, and Third Parties, to Exploit, Antibodies
with affinity for the Antigen that is the subject of the Commercial License, but
raised against another Antigen.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-74-
(c) Except as provided in Section 8.6.1(a) hereof,
notwithstanding anything in this Agreement to the contrary, in the case of each
Commercial License granted by Medarex to Kirin pursuant to Section 8.2.4 hereof,
Medarex hereby retains the right under the Medarex Technology and Medarex's
interests in the KM Patent Rights and KM Know-How, to immunize, and to grant to
its Affiliates, Kirin, and Third Parties rights to immunize, any Collaboration
Mice to raise Antibodies against Antigens for research purposes and to determine
whether Medarex or such licensee or sublicensee, as the case may be, desires to
retain or acquire a license (or a sublicense) with respect to any such
Antibodies or Antigens.
8.3 Kirin Licenses to Medarex.
8.3.1 Breeding License. Subject to Section 8.11 hereof and the other
terms and conditions of this Agreement, Kirin hereby grants to Medarex and
Medarex hereby accepts a non-exclusive, non-transferable (except pursuant to
Section 18.4 hereof), fully paid-up, royalty-free, perpetual (subject to any
rights of termination provided in this Agreement), worldwide right and license,
with rights to sublicense as permitted under Section 8.5.2(a), under the Kirin
Technology and Kirin's interest in the KM Patent Rights and KM Know-How, and any
Kirin Improvements to any of the foregoing, to research, develop, breed or
otherwise make or manipulate, use, import, and export (but not to sell, offer to
sell, lease, offer to lease, or otherwise transfer title to) Collaboration Mice
at any and all of Medarex's (or a Medarex Affiliate's) facilities anywhere in
the world, including, without limitation, subject to Section 8.5.2(a) hereof,
the facilities of a Third Party contract service provider to which Medarex
contracts out the maintenance, propagation, and/or manipulation of Collaboration
Mice in connection with a Services Project. Medarex acknowledges that Article IX
hereof governs the availability and transfer of Kirin Materials that may, but
need not, be used by Medarex and its sublicensees in the enjoyment of the right
and license granted by Kirin to Medarex in this Section 8.3.1.
8.3.2 Evaluation License. Subject to Section 8.11 hereof and the
other terms and conditions of this Agreement, Kirin hereby grants to Medarex,
and Medarex hereby accepts, a non-exclusive, non-transferable (except pursuant
to Section 18.4 hereof), fully paid up, royalty-free, worldwide, perpetual
(subject to any rights of termination provided in this Agreement) right and
license, with rights to sublicense as permitted under Section 8.5.2(b) hereof,
under the Kirin Technology and Kirin's interest in the KM Patent Rights and KM
Know-How, and any Kirin Improvements to any of the foregoing, to immunize
Collaboration Mice to raise Antibodies (and Antibody Materials related thereto)
against, and with affinity for, Antigens, and to use Mice Materials derived from
any Collaboration Mice to generate Antibodies and/or to evaluate such
Antibodies, in each case solely for research purposes (but not for clinical
development), and to determine whether Medarex desires to exercise its rights
under a Reservation License or Commercial License with respect to any such
Antibodies, Antibody Materials related thereto, or Antibody Products containing
such Antibodies, and/or whether any of Medarex's Affiliates, Partners or
In-House Collaborators desires to acquire a sublicense under any such License.
8.3.3 Reservation Licenses. On a Medarex Reservation Target by
Medarex Reservation Target basis, with respect to each Medarex Reservation
Target, subject to Section 8.11 hereof and the other terms and conditions of
this Agreement, Kirin hereby grants to Medarex, and Medarex hereby accepts, a
non-exclusive, non-transferable (except pursuant to Section 18.4 hereof),
royalty-free, worldwide right and license during the Reservation License Period
for such Medarex Reservation Target, with rights to sublicense as permitted
under Section 8.5.2(c) hereof, under the Kirin Technology and Kirin's interest
in the KM Patent Rights and KM Know-How, and any Kirin Improvements to any of
the foregoing, to immunize Collaboration Mice to raise Antibodies (and Antibody
Materials related thereto) against, and with affinity for, such Medarex
Reservation Target, and to use Mice Materials derived from any Collaboration
Mice to generate Antibodies and/or to evaluate such Antibodies, in each case
solely for
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-75-
research purposes (but not for clinical development), and to determine whether
Medarex desires to exercise its rights under a Commercial License with respect
to such Medarex Reservation Target pursuant to Section 4.3 hereof and/or whether
any of Medarex's Affiliates, Partners or In-House Collaborators desires to
acquire a sublicense under any such Commercial License.
8.3.4 Commercial License.
(a) Antigen Exclusive Commercial License. On a Selected
Antibody-by-Selected Antibody basis, with respect to [*] with respect to each
Medarex Exclusive Commercial Target pursuant to Sections 4.4.1(b) and 4.4.2
hereof, subject to Sections 8.3.8(b), 8.3.8(c), 8.3.9(b), 8.3.9(c), and 8.11
hereof and the other terms and conditions of this Agreement, Kirin hereby grants
to Medarex, and Medarex hereby accepts, a worldwide, exclusive (even as to
Kirin), non-transferable (except pursuant to Section 18.4 hereof),
royalty-bearing, perpetual (subject to any rights of termination provided in
this Agreement) right and license, with rights to sublicense as permitted under
Section 8.5.2(d) hereof, under the Kirin Technology and Kirin's interest in the
KM Patent Rights and KM Know-How, and any Kirin Improvements to any of the
foregoing, to Exploit and/or have Exploited in the Field (i) such Selected
Antibody (and Antibody Materials related thereto), and/or (ii) Collaboration
Products containing such Selected Antibody(ies) (and Antibody Materials related
thereto). Kirin represents, warrants and covenants to Medarex that, except as
provided in Section 8.3.8 or 8.3.9 hereof, Medarex shall have rights of
exclusivity as set forth in the preceding sentence and none of Kirin, its
assignees or successors shall grant or shall have granted to any other Person
any license or other right to Exploit and/or have Exploited (which in the case
of past grants shall refer to a license or other right that remains in effect),
(x) Antibodies (and Antibody Materials related thereto) raised against and with
affinity for the Medarex Exclusive Commercial Target against which such Selected
Antibody(ies) was raised, or (y) Antibody Products containing Antibodies (and
Antibody Materials related thereto) raised against and with affinity for such
Medarex Exclusive Commercial Target.
(b) Antigen Non-Exclusive Commercial License. On a Selected
Antibody-by-Selected Antibody basis, with respect to [*] with respect to each
Medarex Non-Exclusive Commercial Target pursuant to Sections 4.4.1(a) and 4.4.2
hereof, subject to Sections 8.3.8(b), 8.3.8(c), 8.3.9(b), 8.3.9(c), and 8.11
hereof and the other the terms and conditions of this Agreement, Kirin hereby
grants to Medarex, and Medarex hereby accepts, a worldwide, exclusive (even as
to Kirin), non-transferable (except pursuant to Section 18.4 hereof),
royalty-bearing, perpetual (subject to any rights of termination provided in
this Agreement), right and license, with rights to sublicense as permitted under
Section 8.5.2(d) hereof, under the Kirin Technology and Kirin's interest in the
KM Patent Rights and KM Know-How, and any Kirin Improvements to any of the
foregoing, to Exploit and/or have Exploited in the Field (i) such Selected
Antibody (and Antibody Material related thereto), and/or (ii) Collaboration
Products containing such Selected Antibody(ies) (and Antibody Materials related
thereto). For the avoidance of doubt, nothing contained in this Section 8.3.4(b)
shall prohibit Kirin from granting to any other Person a license to Exploit
and/or have Exploited Antibodies (and Antibody Materials related thereto), other
than such Selected Antibody(ies), raised against and with affinity for such
Medarex Non-Exclusive Commercial Target.
(c) Antigen Semi-Exclusive Commercial License. On a Selected
Antibody-by-Selected Antibody basis, with respect to [*] with respect to each
Medarex Semi-Exclusive Commercial Target pursuant to Sections 4.4.1(b) and 4.4.2
hereof, subject to Sections 8.3.8(a), 8.3.8(b), 8.3.8(c), 8.3.9(a), 8.3.9(b),
8.3.9(c), and 8.11 hereof and the other terms and conditions of this Agreement,
Kirin hereby grants to Medarex, and Medarex hereby accepts, a worldwide,
exclusive (subject specifically to Sections 8.3.8(a) and 8.3.9(a)) (even as to
Kirin), non-transferable (except pursuant to Section 18.4 hereof),
royalty-bearing, perpetual (subject to any rights of termination provided in
this Agreement), right
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-76-
and license, with rights to sublicense as permitted under Section 8.5.2(d)
hereof, under the Kirin Technology and Kirin's interest in the KM Patent Rights
and KM Know-How, and any Kirin Improvements to any of the foregoing, to Exploit
and/or have Exploited in the Field (i) such Selected Antibody (and Antibody
Material related thereto), and/or (ii) Collaboration Products containing such
Selected Antibody(ies) (and Antibody Materials related thereto). Kirin
represents, warrants and covenants to Medarex that, except as provided in
Section 8.3.8 or 8.3.9 hereof, Medarex shall have rights of exclusivity as set
forth in the preceding sentence and none of Kirin, its assignees or successors
shall grant to any other Person any license or other right to Exploit and/or
have Exploited (which in the case of past grants shall refer to a license or
other right that remains in effect), (x) Antibodies (and Antibody Materials
related thereto) raised against and with affinity for the Medarex Semi-Exclusive
Commercial Target against which such Selected Antibody(ies) was raised, or (y)
Antibody Products containing Antibodies (and Antibody Materials related thereto)
raised against and with affinity for such Medarex Semi-Exclusive Commercial
Target.
8.3.5 Sublicenses Under Kirin In-License Agreements. Subject to
Section 8.11 hereof and the other terms and conditions of this Agreement,
including this Section 8.3.5, with respect to any Kirin Technology that Kirin
Controls pursuant to licenses granted to Kirin by Third Parties in any Kirin
In-License Agreements, if any, the licenses granted by Kirin to Medarex in
Section 8.3.1, 8.3.2, 8.3.3 and 8.3.4 hereof shall include sublicenses under
such Kirin Technology (with the rights for Medarex to grant further sublicenses)
(a) for the same scope and field of use, with the same degree of exclusivity
(i.e., non-exclusive, exclusive, or semi-exclusive), and with respect to the
same Selected Antibodies, Antibody Materials and Collaboration Products as
specified in the relevant license grant in Sections 8.3.1, 8.3.2, 8.3.3 and
8.3.4, as applicable, (b) subject to the same pre-existing grants and retained
rights as apply to the corresponding grants in Sections 8.3.1, 8.3.2, 8.3.3 and
8.3.4 hereof, as applicable, and (c) on the terms and conditions that apply
under the applicable Kirin In-License Agreement, if any, to the exercise of such
sublicense (excluding any payment-related terms thereof).
8.3.6 License Grants Relating to Special Licenses. On a Special
License-by-Special License basis, subject to Section 8.11 hereof and the other
terms and conditions of this Agreement, Kirin hereby grants to Medarex and
Medarex hereby accepts a worldwide, non-transferable (except pursuant to Section
18.4 hereof), royalty-bearing right and license, which license is granted to
Medarex for the sole purpose of permitting Medarex to provide sublicense rights
under such license to the Special Licensee, with rights for the Special Licensee
to grant further sublicenses as provided in the applicable Special License,
under the Kirin Technology and Kirin's interest in the KM Patent Rights and KM
Know-How, and any Kirin Improvements to any of the foregoing, to Exploit and/or
have Exploited Selected Antibodies against Antigens, in each case for the same
scope and field of use, with the same degree of exclusivity with respect to any
such Antigen (i.e., non-exclusive, exclusive, or semi-exclusive), and for the
same license term as applies with respect to the licenses granted by Medarex to
such Special Licensee in the Special License with respect to Patent Rights
Controlled by Medarex and only to the extent that (a) the terms and conditions
of such Special Licenses include within the scope of the licenses or other
rights granted by Medarex to such Special Licensee a license or other grant of
rights with respect to Kirin Mice, Kirin Technology, KM-Mice, KM Patent Rights,
and/or KM Know-How, and (b) in the case of Special License No. 3 and Special
License No. 4, [*] (i) are identified on in a writing delivered by Medarex to
Kirin with respect to this Section 8.3.6 on the date of execution of this
Agreement, and/or (ii) are [*] granted by Medarex to a Special Licensee in
Special License No. 1 or Special License No. 2.
8.3.7 Trademark License Granted by Kirin to Medarex.
(a) Grant. Subject to Section 8.11 hereof and the other
terms and conditions of this Agreement, Kirin hereby grants to Medarex a
limited, worldwide, non-exclusive, non-assignable,
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-77-
royalty-free, right and license during the Term, with rights to sublicense as
permitted under Section 8.5.2(e), to use the Kirin Trademarks and the KM
Trademarks in connection with the Kirin Mice, Kirin Technology, KM-Mice, KM
Patent Rights, KM Know-How, Collaboration Antibodies, and/or Collaboration
Products in accordance with the terms and conditions of this Agreement, in
accordance with Kirin's corporate identity and trademark use guidelines as may
be provided in writing by Kirin to Medarex from time to time, and (i) as
required by applicable law; (ii) as reasonably necessary for the exercise of
rights granted to Medarex pursuant to Section 6.3.1 hereof; or (iii) otherwise
as agreed in writing by Kirin, such agreement not to be unreasonably withheld.
(b) Quality Control. Medarex, upon written request of Kirin,
shall promptly furnish Kirin with representative samples showing use of the
Licensed Trademarks by Medarex (and any of its Affiliates and sublicensees) and,
upon written notification from Kirin, shall cease, or modify in accordance with
Kirin's reasonable instructions (or cause Kirin Affiliates and sublicensees to
cease, or modify in accordance with Kirin's reasonable instructions), any use of
the Licensed Trademarks that Kirin reasonably deems not to be in compliance with
its permitted and reasonable standards and any applicable laws and regulations.
(c) Covenants Relating to Use of Trademarks. Medarex
represents, warrants and covenants that, it will not (i) use any Kirin
Trademarks or any part thereof as part of Medarex's corporate name; (ii) use any
new Trademark confusingly similar to the Kirin Trademarks; or (iii) hereafter
register in any country any Trademark resembling or confusingly similar to the
Kirin Trademarks.
8.3.8 Licenses Subject to Pre-Existing Grants.
(a) Each Medarex Antigen Semi-Exclusive Commercial License
granted by Kirin to Medarex pursuant to Section 8.3.4(c) hereof with respect to
a particular Medarex Semi-Exclusive Commercial Target shall be subject to any
existing grant of licenses, options, or rights of refusal or negotiation, in
each case granted by Kirin prior to the Effective Date under the Kirin
Technology and Kirin's interests in the KM Patent Rights and/or KM Know-How with
respect to one or more Antibodies (which Antibodies in the case of an existing
grant of license shall be listed on the Antibody Sequence List pursuant to
Article II hereof) against the Antigen with respect to which rights are granted
in the Medarex Antigen Semi-Exclusive Commercial License.
(b) Each Commercial License granted by Kirin to Medarex
pursuant to Section 8.3.4 hereof shall be subject to any existing grant of
licenses, options, or rights of refusal or negotiation, in each case granted by
Kirin prior to the Effective Date to Medarex or a Third Party of a license,
options, or rights of refusal or negotiation under the Kirin Technology and
Kirin's interests in the KM Patent Rights and/or KM Know-How to Exploit
Antibodies (and Antibody Materials related thereto) with affinity for the
Antigen (which Antigen in the case of an existing license shall be listed on an
Antigen List pursuant to Article II hereof).
(c) Each Commercial License granted by Kirin to Medarex
pursuant to Section 8.3.4 hereof shall be subject to any existing grant of
licenses, options, or rights of refusal or negotiation, in each case granted by
Kirin prior to the Effective Date to Medarex or a Third Party of a license or
other rights under the Kirin Technology, and Kirin's interests in the KM Patent
Rights and/or KM Know-How to perform research and to determine whether such
licensee desires to acquire a license under such technology with respect to
Antibodies (and Antibody Materials related thereto) raised against and with
affinity for Antigens and/or Antibody Products containing such Antibodies (and
Antibody Materials related thereto).
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-78-
8.3.9 Retained Rights.
(a) Notwithstanding anything in Section 8.3 of this
Agreement to the contrary, in the case of each Medarex Antigen Semi-Exclusive
Commercial License granted by Kirin to Medarex pursuant to Section 8.3.4(c)
hereof, Kirin hereby retains the right to grant licenses, options, or rights of
refusal or negotiation under the Kirin Technology and Kirin's interests in the
KM Patent Rights and KM Know-How to Kirin's Affiliates, Medarex, and Third
Parties, to Exploit one or more Antibodies (and/or Antibody Products containing
such Antibodies) raised against and with affinity for the Antigen that is the
subject of such Antigen Semi-Exclusive Commercial License pursuant to any
agreement entered into by Kirin prior to the date on which such Antigen
Semi-Exclusive Commercial License is first exercised in accordance with the
terms and conditions of Article IV hereof; provided, however, that Kirin does
not retain the rights provided in this Section 8.3.9(a) with respect to any [*]
for such License.
(b) Notwithstanding anything in this Agreement to the
contrary, in the case of each Commercial License granted by Kirin to Medarex
pursuant to Section 8.3.4 hereof, Kirin hereby retains the right to Exploit, and
to grant licenses, options, or rights of refusal or negotiation under the Kirin
Technology and its interest in the KM Patent Rights and KM Know-How to Kirin
Affiliates, Medarex, and Third Parties, to Exploit, Antibodies with affinity for
the Antigen that is the subject of the Commercial License, but raised against
another Antigen.
(c) Except as provided in Section 8.6.1(a) hereof,
notwithstanding anything in this Agreement to the contrary, in the case of each
Commercial License granted by Kirin to Medarex pursuant to Section 8.3.4 hereof,
Kirin hereby retains the right under the Kirin Technology and Kirin's interests
in the KM Patent Rights and KM Know-How, to immunize, and to grant to its
Affiliates, Medarex, and Third Parties rights to immunize, any Collaboration
Mice to raise Antibodies against Antigens for research purposes and to determine
whether Kirin or such licensee or sublicensee, as the case may be, desires to
retain or acquire a license or a sublicense with respect to any such Antibodies
or Antigens.
8.4 Negotiations Regarding Licenses Outside the Field. Each Party
acknowledges that the Licenses granted to the other Party in Sections 8.2 and
8.3 hereof are limited to the Field. The Parties acknowledge and agree that
development and application of Collaboration Products outside the Field may be
possible, and so long as the other Party is performing under and in compliance
with this Agreement, and subject to Section 8.11 hereof, each Party agrees to
negotiate in good faith with the other Party regarding reasonable terms and
conditions, including, without limitation, payment-related terms, that might
apply with respect to any future agreement entered into by the Parties for
applications of Medarex Mice, Medarex Technology, Kirin Mice, Kirin Technology,
KM-Mice, KM Know-How, KM Patent Rights, and/or Collaboration Products outside
the Field.
8.5 Sublicenses.
8.5.1 Sublicense Rights Granted to Kirin.
(a) Sublicense Rights Under Breeding License. Subject to
Section 8.11 hereof, Kirin may grant sublicenses under the license granted to it
in Section 8.2.1 hereof only to a Kirin Affiliate or Third Party contract
service providers (which grant in the case of a Third Party contract service
provider shall be in connection with a Services Project), in each case for the
sole purpose of performing services related to the maintenance, propagation,
and/or manipulation of Collaboration Mice and only with Medarex's prior written
consent, not to be unreasonably withheld; provided, however, that no such
sublicense shall include the right to grant a further sublicense. For purposes
of this
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-79-
Section 8.5.1(a), Medarex hereby consents to Kirin's use of CLEA Japan, Inc. as
a contract service provider for services related to the maintenance,
propagation, and/or manipulation of Collaboration Mice.
(b) Sublicense Rights Under Evaluation License. Subject to
Sections 8.6 and 8.11 hereof, Kirin may grant sublicenses under the Kirin
Evaluation License granted to it in Section 8.2.2 hereof only to its Affiliates,
Partners, In-House Collaborators, and Third Party contract service providers
(which grant in the case of a Third Party contract service provider shall be in
connection with a Services Project); provided, however, that no such sublicense
shall include the right to grant a further sublicense under such Evaluation
License, except that a sublicensee shall have a right to transfer Collaboration
Mice to one or more of its Third Party contract service providers if such Third
Party contract service provider(s) and Kirin enter into an agreement(s) that
governs the terms of such transfer to and use by such Third Party contract
service provider(s).
(c) Sublicense Rights Under Reservation License. Subject to
Sections 8.6 and 8.11 hereof, Kirin may grant sublicenses under any Reservation
License granted to it in Section 8.2.3 hereof only to its Affiliates, Partners,
In-House Collaborators, or Third Party contract service providers (which grant
in the case of a Third Party contract service provider shall be in connection
with a Services Project); provided, however, that with respect to any
immunizations to be performed by the sublicensee using Medarex Mice, Kirin may
transfer Medarex Mice to such sublicensee only in accordance with the terms and
conditions of Article IX hereof. Kirin shall have the right to grant to a
sublicensee under such Reservation License the right to grant a further
sublicense under such Reservation License, provided that, such further
sublicense shall not permit the sublicensee to transfer to any Third Party any
Collaboration Mice, or to grant to any Third Party a license or other right to
use or immunize any Collaboration Mice, except that a sublicensee shall have a
right to transfer Collaboration Mice to one or more of its Third Party contract
service providers if such Third Party contract service provider(s) and Kirin
enter into an agreement(s) that governs the terms of such transfer to and use by
such Third Party contract service provider(s).
(d) Sublicense Rights Under Commercial License.
(i) In General. Subject to Sections 8.5.1(d)(ii),
8.5.1 (e) and 8.11 hereof, Kirin may grant to its Affiliates, Partners, In-House
Collaborators and Third Party contract service providers (which grant in the
case of a Third Party contract service provider shall be in connection with a
Services Project) sublicenses under any Commercial License granted to it in
Sections 8.2.4(a), 8.2.4(b) or 8.2.4(c) hereof; provided, however, that Kirin
shall not grant a sublicense to any such Person under any license (or
sublicense) with respect to [*] and sublicensed to Kirin by Medarex as part of
any such License, which in the case of such [*]; provided, further, that with
respect to any immunizations to be performed by any of Kirin's sublicensees
using Medarex Mice, Kirin may transfer Medarex Mice to such sublicensee only in
accordance with the terms and conditions of Article IX hereof. Kirin shall have
the right to grant to a sublicensee under such Commercial License the right to
grant further sublicenses under such Commercial License, except that such rights
shall expressly exclude the right to grant any sublicense under [*]. The terms
of any such further sublicense shall not permit any such sublicensee to transfer
to any Third Party any Collaboration Mice, or to grant to any Third Party a
license or other right to use or immunize any Collaboration Mice, except that a
sublicensee shall have a right to transfer Collaboration Mice to one or more of
its Third Party contract service providers if such Third Party contract service
provider(s) and Kirin enter into an agreement(s) that governs the terms of such
transfer to and use by such Third Party contract service provider(s).
(ii) Sublicense of [*] Under [*] Agreement.
Notwithstanding anything in this Agreement to the contrary, Medarex [*] licensed
by Medarex to Kirin pursuant to
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-80-
Section 8.2.4 herein shall include [*] any and all [*] to [*] the [*] (with the
exception of [*] or [*]), including without limitation the [*] to [*], or if the
[*] does [*] by [*] of the [*] to [*] a [*] to [*] with respect to [*] and [*]
desires that such Person [*], Medarex [*] that [*] shall [*] a [*] with such
Person [*] agreed to in writing by the Parties on the date of execution of this
Agreement within [*] after the date on which [*] a [*]; provided, however, that
Medarex shall not be required to [*] with respect to any given Antibody or
Antibody Product [*]. If [*] business days after the date on which Medarex
receives a written request from Kirin (which request was properly submitted by
Kirin in accordance with the terms of this Section 8.5.1(d)(ii)), or if [*]
desires to have [*] a [*] with such [*] (as documented by a [*] dated and signed
by such [*], and [*] by [*], which [*] shall be maintained by [*]) but [*] is
subject to [*] from [*] of [*] to enter into a [*], [*] to [*] a [*] with such
Person (which form of agreement [*] agreed to in writing by the Parties on the
date of execution of this Agreement, except that it shall be modified in each
case to indicate the appropriate effective date and the counterparty's name,
address, and other relevant identifying information, and to the extent that
Annex A to the Direct Sublicense Agreement is modifiedYY in accordance with
Section 8.5.1(d)(iii) hereof). In the event that any such Direct Sublicense
Agreement [*], Kirin shall promptly [*]. In the event Medarex enters into [*]
such Direct Sublicense Agreement pursuant to this Section 8.5.1(d)(ii) with a
Person [*], the [*] granted by Medarex to Kirin hereunder shall be [*],
provided, however, that such rights shall be [*] to [*] without [*] by the [*]
in the event and to the extent that the [*].
(iii) [*]. The Parties acknowledge and agree that with
respect to any [*] entered into by Medarex with any Person in accordance with
Section [*], the [*] attached to, and made a part of, such [*] shall be used
during the term of such [*] for the purposes of listing the (A) [*] with respect
to which such Person shall have the license rights set forth in Section [*], (B)
the [*], and (C) the [*] and type(s) of mouse (e.g., HuMAb Mouse(R),
KM-Mousezz(TM), TC Mouse(TM) and/or HAC Mouse(TM)) with respect to which such
Person shall have [*] set forth in Section [*]. It is understood and agreed by
the Parties that prior to any [*] or type(s) of mouse) being added to [*], Kirin
shall have exercised a Commercial License with respect to the Kirin Commercial
Target against which such [*] are raised pursuant to Section 4.3 hereof and
shall have [*] that corresponds to such Commercial License pursuant to Sections
4.4.1 and 4.4.2 hereof, subject to the availability of such Antibody(ies) and
such Antigen(s) as determined in accordance with Article III hereof. From and
after the date on which Kirin has [*] that corresponds to a Commercial License
with respect to a particular Kirin Commercial Target pursuant to and in
accordance with the terms and conditions of Article IV hereof, (1) Kirin may
cause [*] to be amended to include such [*] (and reference to the Antigen
against which such [*] was raised and the [*] and type(s) of mouse covered by
the license) by [*], subject to the agreement of such Person to the terms of
such amendment, in which case Medarex shall execute such an amendment within [*]
business days after receiving Kirin's written request, or (2) such [*] pursuant
to this Section 8.5.1(d)(iii) as of the date on which the terms and conditions
of this Section 8.5.1(d)(iii) hereof are satisfied with respect to the [*],
subject to [*] into such agreement confirming in writing their intent that [*].
In the case of any [*] made pursuant to clause (2) this Section 8.5.1(d)(iii),
within [*] business days after the date on which [*] pursuant to this Section,
Kirin shall deliver to Medarex the [*] (dated and signed by [*]), which shall be
appended to the applicable [*] and made a part thereof. Notwithstanding the
foregoing, Kirin acknowledges and agrees that Medarex shall [*] with respect to
any given Antibody or Antibody Product [*], and Kirin represents, warrants and
covenants to Medarex that Kirin shall not take any action to cause [*] to [*] in
a manner that would [*]. Notwithstanding the foregoing, in the event that Kirin
has not complied with any of the requirements of this Agreement with respect to
the subject matter of any such amendment (including, without limitation, the
listing of the [*] or type(s) of mouse with respect thereto), then such [*]
shall have [*] or [*] and shall be [*] and the [*] shall be [*] with respect to
the subject matter of the [*].
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-81-
(iv) Requests for [*] to Form of [*]. Medarex agrees
that in the event any Person [*] desires to [*] the form of [*], upon [*] will
[*] the form of [*], so long as [*]. Kirin agrees that in the event Medarex [*]
the form of [*], upon Medarex's written request, Kirin will [*] the form of [*],
so long as [*]. For the avoidance of doubt, neither Party shall have any legal
obligation to negotiate with the other Party or any such Third Party, or to [*]
the form of [*], provided that each Party shall perform its obligations under
the first two (2) sentences, as applicable, of this Section. In the event that a
Party agrees, in its sole discretion, to negotiate with the other Party with
respect to any such [*] to the form of [*], the Party requesting the [*] to the
form of [*], upon request by the other Party, shall [*]. Notwithstanding the
foregoing, prior to Medarex's execution of any [*] form of [*], Medarex shall
submit such form of [*] to [*] for [*], which [*].
(e) Sublicense Under [*]. Except with respect to a sublicense
under any [*], for which further sublicensing by Kirin is governed by Section
8.5.1(d)(ii), the sublicenses granted by Medarex to Kirin pursuant to Section
8.2.5 hereof shall include the rights for Kirin to grant further sublicenses
[*]; provided, however, that if any [*] does not permit Kirin to grant further
sublicenses, upon receipt of written notice from Kirin requesting that Medarex
grant a direct sublicense under such Medarex In-License Agreement to a Kirin
Affiliate, Kirin Partner or In-House Collaborator, Medarex and Kirin shall [*]
days after the date on which Kirin so notifies Medarex. From and after the date
on which Medarex and Kirin [*], if Kirin provides additional written requests
for Medarex [*], Medarex [*] within [*] business days after the receipt of any
such request from Kirin. In the event that the Parties at any time during the
Term negotiate and agree upon a form of [*] with respect to [*], once the
Parties have reached agreement on the form of such [*], requests by Kirin or
Medarex for amendments to such form shall be subject to [*] the form of [*].
(f) Sublicense Rights Under Trademark License. Subject to
Section 8.11 hereof, Kirin may grant sublicenses under the Trademark license
granted to it in Section 8.2.6(a) hereof only to its Affiliates, Partners, and
In-House Collaborators, subject to Medarex's prior written consent and provided
that such sublicenses are in accordance with the restrictions, limitations, and
covenants set forth in Section 8.2.6(b) and 8.2.6(c) hereof.
(g) Kirin's Obligations Under Licenses Continue. Each
sublicense granted by Kirin to an Affiliate, Partner, In-House Collaborator, or
Third Party contract service provider pursuant to Section 8.5.1(a), 8.5.1(b),
8.5.1(c), 8.5.1(d), 8.5.1(e), 8.5.1(f) or 8.5.3 hereof shall be consistent with
all the terms and conditions of this Agreement, and subordinate thereto, and
Kirin shall remain responsible to Medarex for the compliance of each sublicensee
with the terms and conditions of this Agreement.
8.5.2 Sublicense Rights Granted to Medarex.
(a) Sublicense Rights Under Breeding License. Subject to
Section 8.11 hereof, Medarex may grant sublicenses under the license granted to
it in Section 8.3.1 hereof only to Medarex Affiliates or Third Party contract
service providers (which grant in the case of a Third Party contract service
provider shall be in connection with a Services Project), in each case, for the
sole purpose of performing services related to the maintenance, propagation,
and/or manipulation of the Kirin Mice or KM-Mice and only with Kirin's prior
written consent, not to be unreasonably withheld; provided, however, that no
such sublicense shall include the right to grant a further sublicense.
(b) Sublicense Rights Under Evaluation License. Subject to
Section 8.11 hereof, Medarex may grant sublicenses under the Medarex Evaluation
License granted to it in Section 8.3.2 hereof only to its Affiliates, Partners,
In-House Collaborators, and Third Party contract service providers (which grant
in the case of a Third Party contract service provider shall be in connection
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-82-
with a Services Project); provided, however, that no such sublicense shall
include the right to grant a further sublicense under such Evaluation License,
except that a sublicensee shall have a right to transfer Kirin Mice or KM-Mice
to one or more of its Third Party contract service providers if such Third Party
contract service provider(s) and Medarex enter into an agreement(s) that governs
the terms of such transfer to and use by such Third Party contract service
provider(s).
(c) Sublicense Rights Under Reservation License. Subject to
Section 8.11 hereof, Medarex may grant sublicenses under any Reservation License
granted to it in Section 8.3.3 hereof only to its Affiliates, Partners, In-House
Collaborators and Third Party contract service providers (which grant in the
case of a Third Party contract service provider shall be in connection with a
Services Project); provided, however, that with respect to any immunizations to
be performed by any sublicensee using Kirin Mice, Medarex may transfer Kirin
Mice to such sublicensee only in accordance with the terms and conditions of
Article IX hereof. Medarex shall have the right to grant to a sublicensee under
such Reservation License the right to grant a further sublicense under such
Reservation License, provided that, such further sublicense shall not permit the
sublicensee to transfer to any Third Party any Kirin Mice or KM-Mice, or to
grant to any Third Party a license or other right to use or immunize any Kirin
Mice or KM-Mice, except that a sublicensee shall have a right to transfer Kirin
Mice or KM-Mice to one or more of its Third Party contract service providers if
such Third Party contract service provider(s) and Medarex enter into an
agreement(s) that governs the terms of such transfer to and use by such Third
Party contract service provider(s).
(d) Sublicense Rights Under Commercial License. Subject to
Section 8.11 hereof, Medarex may grant sublicenses under any Commercial License
granted to it in Sections 8.3.4(a), 8.3.4(b), or 8.3.4(c) hereof only to its
Affiliates, Partners, In-House Collaborators, and Third Party contract service
providers (which grant in the case of a Third Party contract service provider
shall be in connection with a Services Project); provided, however, that with
respect to any immunizations to be performed by any sublicensee using Kirin
Mice, Medarex may transfer Kirin Mice to such sublicensee only in accordance
with the term and conditions of Article IX hereof. Medarex shall have the right
to grant to a sublicensee under such Commercial License the right to grant
further sublicenses under such Commercial License. The terms of any such further
sublicense shall not permit the sublicensee to transfer to any Third Party any
Kirin Mice or KM-Mice, or to grant to any Third Party a license or other right
to use or immunize any Kirin Mice or KM-Mice, except that a sublicensee shall
have a right to transfer Kirin Mice or KM-Mice to one or more of its Third Party
contract service providers if such Third Party contract service provider(s) and
Medarex enter into an agreement(s) that governs the terms of such transfer to
and use by such Third Party contract service provider(s).
(e) Sublicense Rights Under Trademark License. Subject to
Section 8.11 hereof, Medarex may grant sublicenses under the Trademark license
granted to it in Section 8.3.7(a) hereof only to its Affiliates, Partners, and
In-House Collaborators, subject to Kirin's prior written consent and provided
that such sublicenses are in accordance with the restrictions, limitations, and
covenants set forth in Section 8.3.7(b) and 8.3.7(c) hereof.
(f) Medarex's Obligations Under Licenses Continue. Each
sublicense granted by Medarex to an Affiliate, Partner, In-House Collaborator or
Third Party contract service provider pursuant to Section 8.5.2(a), 8.5.2(b),
8.5.2(c), 8.5.2(d), 8.5.2(e) or 8.5.3 hereof shall be consistent with all the
terms and conditions of this Agreement, and subordinate thereto, and Medarex
shall remain responsible to Kirin for the compliance of each sublicensee with
the terms and conditions of this Agreement.
8.5.3 Sublicenses to Affiliates to Enter into Agreements in Place of
a Party.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-83-
(a) Right to Sublicense to Affiliates. Subject to this Section
8.5.3, Section 8.11 and the other terms and conditions of this Agreement, in
addition to the sublicense rights provided in Sections 8.5.1 and 8.5.2 hereof,
as applicable, each Party hereto may grant sublicenses under the rights and
licenses granted to it pursuant to Section 8.2 or Section 8.3, as applicable, to
any of its Affiliates for the purposes of such Affiliate entering into Project
Agreements governing In-House Projects and/or Partner Projects (and, in the case
of Medarex, HuMAb License Projects, HuMAb Collaboration Projects and/or other
projects relating to HuMAb Projects, to the extent that this Agreement contains
terms and conditions applicable to such agreements) with other Affiliates or
Third Parties in place of such Party. Notwithstanding the foregoing, Kirin's
right to grant sublicenses to a Kirin Affiliate(s) under any Commercial License
granted to Kirin by Medarex shall remain subject to Section 8.5.1(d)(i) and (ii)
hereof, and if necessary in order for Kirin to provide rights under such
Commercial License to a Kirin Affiliate, Medarex shall enter into a Direct
Sublicense Agreement with such Affiliate pursuant to the terms and conditions of
Section 8.5.1(d)(ii) hereof. In the event that a Party grants to an Affiliate
(a) a sublicense under the Breeding License granted to such Party in Section
8.2.1 or 8.3.1 hereof, as applicable, and/or (b) in accordance with Article IX
hereof, rights for such Person to transfer Kirin Mice or KM-Mice or Mice
Materials derived therefrom (in the case of rights granted by Medarex to a
Medarex Affiliate) or rights to transfer Medarex Mice or KM-Mice or Mice
Materials derived therefrom (in the case of rights granted by Kirin to a Kirin
Affiliate), and the Person subsequently becomes a Former Affiliate, such Party
shall terminate such sublicense and such rights to transfer such mice and Mice
Materials.
(b) Form of Agreement with Affiliates. With respect to each
agreement whereby a Party grants to an Affiliate a sublicense pursuant to
Section 8.5.3(a) above, such agreement shall (i) be consistent with all the
terms and conditions of this Agreement, and (ii) provide that such Affiliate
shall be bound by the same terms and conditions applicable to such Party as set
forth in this Agreement with respect to entering into and performing under
Project Agreements governing In-House Projects and/or Partner Projects (and, in
the case of Medarex, HuMAb License Projects, HuMAb Collaboration Projects and/or
other projects relating to HuMAb Projects, to the extent that this Agreement
contains terms and conditions applicable to such agreements) including, without
limitation, the availability and selection of Antigens and Antibodies for the
exercise of rights and licenses as determined in accordance with this Agreement.
(c) Notices Relating to [*]. During the Term, in the event that
Medarex [*], or (ii) secures by contract the right to designate [*], and in
either case such information is publicly disclosed in a press release issued by
Medarex, Medarex shall provide to Kirin a copy of such press release; provided,
however, that Medarex's [*] to provide to Kirin a copy of such press release
in accordance with this Section 8.5.3(c) shall [*] of Medarex's [*] under this
Agreement.
8.6 Covenants Relating to Licenses and Sublicenses.
8.6.1 Covenants Relating to Evaluation Licenses and Sublicense(s)
Under Evaluation Licenses.
(a) Covenants Relating to Antigens Determined to be [*]
(i) In the event that an Antigen is determined to be [*]
to Medarex in accordance with the terms and conditions of Section [*] hereof, or
is determined to be [*] to Kirin in accordance with the terms and conditions of
Section [*] hereof, and such Antigen is the subject of a grant of a license or
other rights by a Party to a Third Party pursuant to a written agreement entered
into by the Party and the Third Party, the Party that made an availability
inquiry with respect to such Antigen
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-84-
pursuant to either of such Sections shall [*] to raise Antibodies against such
Antigen, unless and until (x) such Antigen is [*] in accordance with the terms
and conditions of Section [*] or [*] hereof; or (y) the Party that [*] or other
[*] with respect to such Antigen [*] (and in the case where such Party is [*]
that [*], is [*] by the [*] to [*], and in fact [*] in a [*] to the [*]) that
the [*] of the [*] with respect to such Antigen is [*] the [*] or other [*].
(ii) Medarex and Kirin each further covenants to the
other that it will include in its Project Agreements governing Partner Projects
and/or In-House Projects entered into after the Effective Date terms and
conditions that require such Party's Affiliates, Partners and In-House
Collaborators, as applicable, either (x) [*] any Collaboration Mice to immunize
against an Antigen [*] is [*] hereto that such Antigen is [*] (as determined in
accordance with the terms and conditions of Section [*] or [*] hereof, as
applicable) or (y) [*] any Collaboration Mice to immunize against an Antigen [*]
is [*] hereto that such Antigen is [*] (as determined in accordance with the
terms and conditions of Section [*] or [*] hereof, as applicable) [*]; provided,
however, that with respect to clause (y) hereof, [*] each [*] that [*] a Party
to a [*], as applicable, [*] (A) such Antigen is [*] in accordance with the
terms and conditions of Section [*] or [*] hereof in response to [*]; or (B) the
Party that has [*] such Antigen [*] (and in the case where such Party is [*], is
[*] by the [*], and in fact [*] to the [*]) that either (1) such Party has [*]
to a [*] or other [*] with respect to such Antigen pursuant to [*] or (2) if
such Party has [*] to a [*] with respect to such Antigen pursuant to [*], the
[*] with respect to such Antigen is [*] or other [*] to such [*].
(b) Covenants Relating to Sublicense Under Evaluation License.
(i) Each Party covenants to the other Party that,
subject to Section 8.6.1(b)(iv), with respect to each sublicense under its
Evaluation License that is granted by such Party to a Third Party pursuant to an
agreement originally entered into on or after the Effective Date, such Party
shall [*] such Third Party to [*] terms and conditions [*] of this Section as
follows: (1) such Party shall [*] terms and conditions [*] from [*],
Collaboration Antibodies raised in the exercise of such sublicense, or
Collaboration Products containing such Collaboration Antibodies, unless and
until such [*] a [*] a [*] or a [*] (and/or in the case of a [*], a [*]) with
respect to the Antigen against which such Collaboration Antibodies are raised;
(2) in the event such Party is [*] in its [*] to [*] terms and conditions [*]
clause (1), such Party shall [*] terms and conditions [*] to either [*] and any
[*] or to [*] to the Party that grants such sublicense any and all [*] of such
[*] any such [*], such Collaboration Antibodies, or Collaboration Products
containing such Collaboration Antibodies, in the event that [*] does not [*] a
[*] or a [*] (and/or, in the case of a [*], a [*] with respect to the Antigen
against which such Collaboration Antibodies are raised within the [*] the terms
of the sublicense agreement; and (3) in the event such Party is [*] in it [*]
terms and conditions [*] either clause (1) or (2) above, such Party shall [*]
terms and conditions [*] to [*] to such Party a [*], with the [*] to [*]
Collaboration Antibodies (and Collaboration Products containing such
Collaboration Antibodies) raised in the exercise of such [*] against and with
affinity for an Antigen other than an Antigen with respect to which the [*] a
[*] or a [*] (and/or, in the case of a [*], a [*]) within the [*] the terms of
the sublicense agreement.
(ii) In the event that a Party grants to a Third Party
a sublicense under its Evaluation License pursuant to an agreement originally
entered into on or after the Effective Date, and [*], such Party [*],
Collaboration Antibodies raised in the exercise of such sublicense, and/or
Collaboration Products containing such Collaboration Antibodies, and the
sublicensee [*] (and/or, in the case of a sublicensee [*]) to [*] and/or [*]
either such Collaboration Antibodies (and/or Collaboration Products containing
such Collaboration Antibodies) or other Antibodies (whether or not Collaboration
Antibodies) (and/or Antibody Products containing such Antibodies) raised against
and with affinity for
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-85-
the same Antigen within the [*] the terms of such sublicense agreement, such
Party shall (and hereby does), [*] and subject to such other Party's acceptance
of, and agreement to comply with, any terms that apply to the exercise of such
rights by the first Party and its sublicensees (including any economic terms
relating to the exercise of such sublicense), grant to such other Party a
non-exclusive, perpetual, worldwide, sublicense under such license in the Field,
with a right to grant further sublicenses (which sublicense shall be [*] in the
event that such first Party obtains such license on a [*] basis); provided,
however, that the grant of a sublicense by a Party to the other Party in this
Section 8.6.1(b)(ii) shall be deemed [*] any Collaboration Antibodies (x) raised
against and with affinity for an Antigen with respect to which the [*], (y)
raised against and with affinity for an Antigen against which the [*], or (z) in
the case of [*], raised against and with affinity for an Antigen [*] in
connection with [*] (in each case as reflected on the appropriate Antigen List).
(iii) It is understood and agreed that the intent of
Sections 8.6.1(b)(i) and 8.6.1(b)(ii) hereof is to [*] a Party (and/or its
sublicensees) with [*] to [*] certain Antibodies (and Antibody Products
containing such Antibodies) raised against and with affinity for an Antigen in
connection with the exercise of the licenses granted to such Party in Article
VIII hereof and sublicenses granted by such Party hereunder (and, in the case of
Medarex, through the exercise of Medarex's rights under the Medarex Technology)
in the event that [*] with respect to Antibodies raised against such Antigen.
Notwithstanding anything contained in this Section 8.6.1(b), it is understood
and agreed that such Section shall [*], and that [*] this Section 8.6.1(b) is
[*] that consist solely of the [*].
(iv) Notwithstanding anything contained in Section
8.6.1(b)(i) hereof, a Party may grant to a Third Party a sublicense under its
Evaluation License in an agreement originally entered into on or after the
Effective Date, [*], provided that the Party granting such sublicense (A) within
[*] business days of the grant of such sublicense provides notice thereof to the
other Party and (1) indicates in such notice that it is [*] upon the giving of
such notice, or (2) pays to such other Party the amount of [*] in connection
with such grant (which amount shall [*]; or (B) [*] the inclusion in the
agreement with the Third Party of terms and conditions that [*] Each Party shall
be permitted during the Term to exercise, in the aggregate, [*].
8.6.2 Covenants Relating to Sublicense(s) Under Reservation
License(s).
(a) Each Party covenants to the other Party that with respect
to each sublicense under any Reservation License that is granted by such Party
to a Third Party pursuant to an agreement originally entered into on or after
the Effective Date, such Party shall [*] include in such agreement a requirement
that the sublicensee grant to such Party [*] license, with [*], in the [*]
Collaboration Antibodies raised [*], in the event that the sublicensee does [*]
(and/or, in the case of a sublicensee [*]) to [*] and/or [*] either such
Collaboration Antibodies (and/or Collaboration Products containing such
Collaboration Antibodies) or other Antibodies (whether or not Collaboration
Antibodies) (and/or Antibody Products containing such Antibodies) raised against
and with affinity for the same Antigen within the [*] the terms of such
sublicense agreement.
(b) In the event that a Party grants to a Third Party a
sublicense under its Reservation License pursuant to an agreement originally
entered into on or after the Effective Date, and [*], such Party obtains from
such Third Party a license (with a right to sublicense) [*], and the sublicensee
does [*] (and/or, in the case of a sublicensee [*]) to [*] and/or [*] either
such Collaboration Antibodies (and/or Collaboration Products containing such
Collaboration Antibodies) or other Antibodies (whether or not Collaboration
Antibodies) (and/or Antibody Products containing such Antibodies) raised against
and with affinity for the same Antigen within the [*] the terms of such
sublicense agreement, such Party shall (and hereby does), [*] and [*], any terms
that apply to the exercise of such rights by the first Party
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-86-
and its sublicensees (including any economic terms relating to the exercise of
such sublicense), grant to such other Party a [*]; provided, however, that the
grant of a sublicense by a Party to the other Party in this Section 8.6.2(b)
shall be deemed [*] Collaboration Antibodies (x) raised against and with
affinity for an Antigen with respect to which the [*], (y) raised against and
with affinity for an Antigen against which the [*], or (z) [*], raised against
and with affinity for an Antigen [*].
(c) It is understood and agreed that the intent of Sections
8.6.2(a) and 8.6.2(b) hereof is to [*] a Party (and/or its sublicensees) with
[*] to [*] and/or have [*] certain Antibodies (and Antibody Products containing
such Antibodies) raised against and with affinity for an Antigen in connection
with the exercise of the licenses granted to such Party in Article VIII hereof
and sublicenses granted by such Party hereunder (and, in the case of Medarex,
through the exercise of Medarex's rights under the Medarex Technology) in the
event that [*] or other [*] with respect to Antibodies raised against such
Antigen. Notwithstanding anything contained in this Section 8.6.2, it is
understood and agreed that such Section shall [*], and that [*] this Section
8.6.2 is [*] that consist solely of the [*].
8.6.3 Covenants Relating to Sublicense(s) Under Evaluation License,
Reservation License and/or Commercial License. Each Party covenants that, with
respect to any of the following sublicenses granted by such Party pursuant to an
agreement entered into on or after the Effective Date under the Evaluation
License granted to it pursuant to Section 8.2.2 or 8.3.2 hereof, a Reservation
License granted to it pursuant to Section 8.2.3 or 8.3.3 hereof, as the case may
be, or a Commercial License granted to it pursuant to Section 8.2.4 or 8.3.4
hereof, as the case may be:
(a) Improvements to Collaboration Mice. The terms and
conditions of such sublicense agreement that relate to the exercise of such
sublicense shall (i) provide that any such sublicenses granted to the
sublicensee do not permit the sublicensee to breed or otherwise attempt to
improve the Collaboration Mice, and (ii) require the sublicensee to assign to
the licensor Party any and all of the sublicensee's rights, title and interest
in and to any Improvements to any Collaboration Mice that are conceived or
developed by or on behalf of the sublicensee in connection with use of any
Collaboration Mice and in violation of such sublicensee's rights under the
sublicense; provided, however, that in the event that [*], the terms and
conditions of such sublicense agreement shall [*]. For the avoidance of doubt,
nothing contained in this Section shall prohibit Medarex from granting to an
Affiliate or Third Party a right to breed or otherwise improve the Medarex Mice.
(b) Destruction of Mice, Antibodies and Antibody Products.
Such Party shall [*] in such sublicense agreement (a) a requirement that, within
a reasonable time after the expiration of the applicable license period, such
sublicensee shall destroy all Collaboration Mice immunized with an Antigen(s)
pursuant to such license, all materials derived from such Collaboration Mice,
and all Antibodies and Antibody Products obtained through use of such
Collaboration Mice with respect to such Antigen(s), if as of the date of
expiration or termination of the applicable license period no further license,
sublicense, or other rights under the Medarex Technology, Kirin Technology, or
KM Patent Rights have been granted to such sublicensee and remain in effect, and
(b) a requirement that promptly after any such destruction an agent of such
sublicensee shall provide such Party with written certification thereof.
8.6.4 Covenants Relating to Patent Rights, Know-How and Improvements
Controlled by Affiliates. The Parties hereto acknowledge and agree that during
the Term a Party may elect to grant sublicenses to Affiliate(s) (consistent with
the terms and conditions of this Agreement, including Section 8.5.3 hereof) or
otherwise provide to its Affiliate(s) access to its Technology, the KM Patent
Rights, KM Know-How or one or more of the Collaboration Mice (in each case
consistent with the terms and conditions of this Agreement, including Section
8.5.3 hereof). In the event that a Person which is an Affiliate of a Party
within the meaning of Section 1.8 hereof (but for purposes of this Section
8.6.4,
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-87-
not a Person which is a Former Affiliate of a Party within the meaning of
Section 1.72 hereof), in connection with the exercise of a license or sublicense
in or to Technology or KM Patent Rights or KM Know-How, or otherwise as result
of having access to any Collaboration Mice, (a) invents or otherwise Controls
any Information and Inventions that would constitute Know-How of a Party if such
Party invented or otherwise Controlled any such Information or Inventions, (b)
invents or otherwise Controls any Improvements to any Collaboration Mice that
would constitute Medarex Improvements or Kirin Improvements, if such
Improvements were Controlled by Medarex or Kirin, as the case may be, or (c)
Controls any Patent Rights that would constitute Medarex Patent Rights, Kirin
Patent Rights or KM Patent Rights if such Patent Rights were Controlled by
Medarex and/or Kirin, as the case may be, then in each case the Party for which
such Person is an Affiliate shall obtain from such Affiliate rights with respect
to such Know-How, Improvements and Patent Rights such that such Party Controls
such Know-How, Improvements and Patent Rights for purposes of this Agreement.
For the avoidance of doubt, the Party for which such Person is an Affiliate (y)
shall obtain such rights from such Person with respect to any such Know-How,
Improvements or Patent Rights invented or originally Controlled by such Person
during the Period when such Person satisfies the definition of an "Affiliate"
contained in Section 1.8 hereof (but not the definition of a "Former Affiliate"
contained in Section 1.72 hereof), which rights once obtained from such Person
shall continue to exist in favor of such Party even in the event that such
Person later becomes a Former Affiliate, and (z) shall have no obligation to
obtain such rights from such Person with respect to any such Know-How,
Improvements or Patent Rights invented or otherwise originally Controlled by
such Person during the period when such Person satisfies the definition of a
"Former Affiliate". Notwithstanding the foregoing, nothing in this Section 8.6.4
shall affect any obligation of a Party arising under Section 8.6.3 hereof, with
respect to certain sublicense agreements entered into by such Party, including
sublicense agreements entered into by such Party and an Affiliate or Former
Affiliate.
8.6.5 Covenant Relating to Refusal Rights. Each Party represents,
warrants and covenants to the other Party that from and after the Effective
Date, it shall not enter into a Project Agreement with an Affiliate or Third
Party in which such Party grants to the Affiliate or Third Party Refusal Rights;
provided, however, that nothing contained in this Section 8.6.5 shall [*]
granting to an Affiliate or Third Party [*] to [*] or, in the case of [*], in
each case, with respect to one or more Antibodies raised against and with
affinity for an Antigen.
8.7 Termination of Commercial License.
8.7.1 Termination. Either Party may terminate any Commercial License
granted to it hereunder by the other Party on a country-by-country basis, with
respect to any Commercial Target, at any time with immediate effect by giving
written notice to the other Party, or if the giving of such notice shall not be
permitted under applicable law, either by any clear and unequivocal
manifestation of such intention that is permitted by applicable law or by
unilateral surrender or nonuse of the applicable rights in any commercially
reasonable manner under the circumstances (such Party terminating or attempting
to terminate as provided herein, is herein called the "Terminating Party" and
the other Party is sometimes referred to as the "Non-Terminating Party").
Following such complete or partial termination of the particular license, the
terminating Party shall have no further right, title, or license under the other
Party's Technology or the other Party's interest in the KM Patent Rights or KM
Know-How, to the extent of the termination of such License.
8.7.2 Covenants. Upon termination (or attempted termination) of any
Commercial License granted hereunder, in whole or in part, pursuant to Section
8.7.1 hereof or by rejection pursuant to the Bankruptcy Code or by rescission
pursuant to the Japanese bankruptcy law, in the event that the Terminating Party
(or any of its sublicensees) has filed any patent applications containing a
novel
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-88-
disclosure of, or claiming, Information or Inventions containing the other
Party's Technology or KM Patent Rights or KM Know-How and relating to or
covering Collaboration Antibodies or Collaboration Products that are within the
scope of the terminated portion of the license, the Parties intend for the
licensor Party to have freedom to operate with respect to such Information and
Inventions, so long as the licensor Party is in compliance with its obligations
under this Agreement to the licensee Party. The Terminating Party hereby grants,
and in the case of any such patent application filed by the Terminating Party's
sublicensee (in connection with an agreement entered into by the Terminating
Party and the sublicensee after the Effective Date), [*] a non-exclusive,
sublicensable, transferable license in the Field under such Patent Rights, [*]
(and with respect to any such license granted to the Terminating Party by a
sublicensee, the Terminating Party hereby grants to the Non-Terminating Party a
non-exclusive sublicense under the license granted to the Terminating Party by
its sublicensee, subject to the Non-Terminating Party's acceptance of and
agreement with any terms and conditions that apply to the exercise of such
rights by the Terminating Party or its sublicensees [*])); provided, however,
that the Terminating Party hereby grants such license pursuant to this Section
8.7.2 and only with respect to claims relating to or covering subject matter
containing the Non-Terminating Party's Technology and the Non-Terminating
Party's interest in KM Patent Rights and relating to or covering Collaboration
Antibodies or Collaboration Products that are within the scope of the terminated
portion of the license (and only to the extent not prohibited by the terms of
any Third Party agreement pursuant to which such Party Controls any such Patent
Rights). Notwithstanding the foregoing, it is understood and agreed that nothing
contained in this Section 8.7.2 is intended to require a Party or its
sublicensee to grant any license under Patent Rights in any Information and
Inventions that consist solely of the composition of an Antigen or a Production
Process Development.
8.8 License Grants and Exercise [*].
8.8.1 Special Licenses. The Parties acknowledge and agree that,
[*], Medarex granted to Special Licensees certain rights, including a
license(s), in or to certain technology owned or in-licensed by Medarex, and
that not all the terms and conditions relating to such Special Licensees'
Exploitation of such technology mirror the terms and conditions hereunder
relating to Exploitation of licensed technology by Medarex's sublicensees.
Medarex acknowledges and agrees that it has reviewed Special License No. 1 and
Special License No. 2, and Kirin acknowledges and agrees that it has reviewed
redacted versions of such agreements, and based on their respective reviews of
those agreements, each Party hereby agrees that any and all material conflicts
between Special License No. 1, on the one hand, and this Agreement, on the other
hand, and between Special License No. 2, on the one hand, and this Agreement, on
the other hand, are described in a writing prepared jointly by the Parties with
respect to this Section 8.8.1 and exchanged by the Parties on the date of
execution of this Agreement. With respect to any material conflicts noted on the
writing exchanged by the Parties pursuant to this Section 8.8.1, and with
respect to any other conflicts that may be determined in the future to exist
between either Special License No. 1 or Special License No. 2 and this
Agreement, the terms of the Special License No. 1 and/or Special License No. 2,
as the case may be, shall control. In addition, Medarex acknowledges and agrees
that it has reviewed Special License No. 3 and Special License No. 4 (which
agreements have been reviewed by Kirin only in their redacted forms as available
to the public from the SEC's database), and based on Medarex's review of those
agreements, Medarex hereby agrees that there are no material conflicts between
Special License No. 3, on the one hand, and this Agreement, on the other hand,
and between Special License No. 4, on the one hand, and this Agreement, on the
other hand, that are materially different than any conflicts contained in
Special License No. 1 and/or Special License No. 2 (whether or not listed on the
writing exchanged by the Parties pursuant to this Section 8.8.1). With respect
to any material or other conflicts that may be determined in the future to exist
between Special License No. 3, on the one hand, and this Agreement, on the other
hand, and/or between Special License No. 4, on the one hand, and this Agreement,
on the other hand, which conflicts are not materially different than any
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-89-
conflicts that exist between Special License No. 1 and/or Special License No. 2,
on the one hand, and this Agreement, on the other hand, the terms of Special
License No. 3 and Special License No. 4 shall control. With respect to any
material conflicts that may be determined in the future to exist between either
Special License No. 3, on the one hand, and this Agreement, on the other hand,
and/or between Special License No. 4, on the one hand, and this Agreement, on
the other hand, which [*] that exist between Special License No. 1 and/or
Special License No. 2, on the one hand, and this Agreement, on the other hand,
[*].
8.8.2 License Grants in Connection with Partner Projects. The
Parties acknowledge and agree that, [*] Kirin has, under a single Project
Agreement governing a Kirin Partner Project, granted to Corixa Corporation
licenses under the Kirin Technology and/or sublicenses under the Medarex
Technology, KM Patent Rights, or KM Know-How to immunize Collaboration Mice
and/or to Exploit and/or have Exploited Collaboration Antibodies (or
Collaboration Products containing such Collaboration Antibodies) against one or
more Antigens. The Parties acknowledge and agree that, [*], Medarex [*] to one
or more Partners sublicenses or options, or rights of refusal or negotiation
under the Kirin Technology, KM Patent Rights, or KM Know-How to Exploit and/or
have Exploited Collaboration Antibodies (and/or Collaboration Products
containing such Collaboration Antibodies) against one or more Antigens;
provided, however, that such acknowledgment and agreement by the Parties shall
in no way [*] of any rights afforded to a Party hereto or of any covenants made
by either Party or a release of obligations imposed on either Party under this
Agreement with respect to grants by a Party to Partners [*]. Subject to the
availability of such Collaboration Antibodies and Antigens for licensing, any
such license or other rights granted by Kirin or Medarex shall be effective as
of the date of such grant of rights and shall remain in full force and effect as
if such grants were made pursuant to this Agreement. (For the avoidance of
doubt, the payment obligations of the Parties hereunder shall apply with respect
to such agreements). From and after the Effective Date, each Party shall grant
sublicenses to its Partners under the other Party's Technology and the other
Party's rights and interest in and to the KM Patent Rights and KM Know-How only
in accordance with the terms and conditions of this Agreement.
8.8.3 Exercise of Rights Pursuant to [*] for Collaboration in
Connection with In-House Projects. The Parties acknowledge and agree that, [*],
Kirin has exercised certain rights under the license granted to Kirin by Medarex
in connection with a Kirin In-House Project, and Medarex has exercised certain
rights granted to Medarex by Kirin in connection with Medarex In-House Projects,
and that any such rights shall be deemed to have been granted by the licensor
Party and exercised by the licensee Party on a non-exclusive basis under an
Evaluation License; provided, however, that with respect to each Target
designated by Kirin as an In-House Target [*], such Target shall be deemed to be
a Reservation Target and a ROFR Target and Kirin shall be deemed to have
exercised a Kirin Reservation License with respect to such Target, in each case
as of the Designation Date for such Reservation Target, and to have renewed such
Reservation License every [*] months during the period from the [*] such that as
of [*] Kirin shall continue to have a Reservation License with respect to such
Target, which Reservation License shall expire on the date that is [*] months
after the [*] renewal date unless otherwise renewed by Kirin in accordance with
the terms and conditions of Section 4.2.4 hereof (such period for each such
Reservation License shall be deemed to be the "Reservation License Period").
From and after the Effective Date, each Party shall continue to exercise rights
in and to the other Party's Technology and the other Party's rights and interest
in the KM Patent Rights and KM Know-How in connection with In-House Projects
only in accordance with the terms and conditions of this Agreement.
8.9 Condition Precedent to Grant of a Commercial License. Notwithstanding
anything contained in this Agreement, any grant by Medarex to Kirin or by Kirin
to Medarex of a Commercial License, and any exclusive license granted by Kirin
to Medarex pursuant to Section 8.3.6 hereof, shall be conditioned on the
termination or expiration of the applicable waiting period with respect to such
License, if any, under the ▇▇▇▇-▇▇▇▇▇-▇▇▇▇▇▇ Antitrust Improvements Act of 1976,
as amended, 15 U.S.C. (S) 18a.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-90-
8.10 Registration of Patent Licenses. Kirin acknowledges and agrees that,
subject to Section 8.11 hereof, Medarex may register with the Japanese Patent
Office, to the full extent permitted by Japanese law, each of the license grants
made to Medarex by Kirin in this Agreement. Kirin warrants and covenants to
Medarex that, subject to Section 8.11 hereof, Kirin shall cooperate fully with
Medarex to register such license grants with the Japanese Patent Office,
including by executing and consenting to the filing of such documents as are
reasonably required to accomplish such registration(s).
8.11 [*] Obligations and Rights Preserving Excuse.
8.11.1 Acknowledgement of Intention of Parties. The Parties hereto
acknowledge and agree that each Party owes the other performance of a variety of
obligations under this Agreement and that the performance of those obligations
is given in consideration of reciprocal obligations owed by the other Party. The
Parties therefore agree that it is their intention that should a Party (the
"Obligor Party") [*] to the other Party (the "Receiving Party") to such an
extent that the Receiving Party is [*] under this Agreement, then the Obligor
Party should not be entitled to [*] during the period of the Obligor Party's [*]
owed by the Receiving Party to the Obligor Party. The intention is that [*]
Obligor Party should be entitled to [*] of this Agreement from the Receiving
Party without [*] owed to the Receiving Party because a substantial part of the
consideration each Party is receiving in exchange for what it is providing, is
the [*] by the other Party. Therefore, the Parties agree that in the event an
Obligor Party [*] under this Agreement to such an extent that the Receiving
Party is [*] under this Agreement, then regardless of whether the Obligor Party
has a [*], including any [*] on the use by the Receiving Party of [*] from the
Obligor Party, such circumstance shall give rise to a condition referred to in
this Agreement as "Rights Preserving Excuse" in favor of the Receiving Party. If
a Receiving Party has "Rights Preserving Excuse" it shall mean that the
Receiving Party is [*] to the Obligor Party under this Agreement, including with
respect to this Article VIII, in the event that a Cure is not effectuated by the
Obligor Party within the Cure Period, all within the meaning of this Section
8.11. One example of a circumstance that would give rise to Rights Preserving
Excuse, but not the sole example, is where the Obligor Party has become [*]
under this Agreement such that the Receiving Party is [*] under this Agreement,
and should itself therefore be [*] under the terms of this Agreement from [*] to
the Obligor Party. Further, to the extent permitted by applicable law, the
Parties intend for the Receiving Party to receive the same benefits under this
Section 8.11 whether the Obligor Party is seeking protection under the [*] laws
of the United States or the [*].
8.11.2 Mechanics of Assertion of Rights Preserving Excuse. In the
event that circumstances have arisen to cause the Receiving Party to reasonably
conclude that it has Rights Preserving Excuse, then:
(a) if there is [*] on the part of the Receiving Party to
[*] from the Obligor Party and/or to notify the Obligor Party that the Receiving
Party believes it has Rights Preserving Excuse, then the Receiving Party shall
send the Obligor Party written notice that Rights Preserving Excuse has arisen,
providing a reasonable description of the basis for the assertion of Rights
Preserving Excuse, and the Obligor Party shall have [*] calendar days within
which either to advise the Receiving Party the means by which a Cure shall be
effected by the Obligor Party within the Cure Period (as defined below), or
advise the Receiving Party that the Obligor Party believes no Rights Preserving
Excuse has arisen and why; or
(b) if there is [*] on the part of the Receiving Party to
[*] from the Obligor Party and/or to notify the Obligor Party that the Receiving
Party believes it has Rights Preserving Excuse, including by [*], then the
Receiving Party shall file a pleading, paper or other notice with the applicable
court [*] or, in the case of a [*], stating that Rights Preserving Excuse has
arisen and seeking to compel any performance that the Receiving Party believes
has [*], and providing a reasonable description of the
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-91-
basis for the assertion of Rights Preserving Excuse. The filing of the pleading,
paper or other notice with the court [*] as described herein shall constitute
notice to the Obligor Party of the Receiving Party's claim that Rights
Preserving Excuse has arisen, regardless of how or whether any motion,
proceeding or matter is resolved pursuant to the pleading, paper or other notice
that was filed with the applicable court [*].
8.11.3 [*] If, after the Receiving Party gives notice of a Rights
Preserving Excuse pursuant to Section 8.11.2(a) or 8.11.2(b) hereof (a "Notice
of Rights Preserving Excuse"), (i) the Obligor Party proposes a Cure to the
Receiving Party in writing and does not receive, within [*] calendar days
thereafter, written confirmation from the Receiving Party that the proposed Cure
is acceptable to the Receiving Party, or (ii) the Obligor Party disputes whether
Rights Preserving Excuse has arisen in favor of the Receiving Party, then the
Obligor Party may seek to have issues of whether the Obligor Party has proposed
an adequate Cure and/or whether a Rights Preserving Excuse exists resolved by
[*]. If the Obligor Party desires to have any such issue so resolved, the
Obligor Party shall so notify the Receiving Party with a written [*]." Upon
receipt by the Receiving Party of the [*], the Parties shall endeavor to agree
on the choice of a cost-effective [*] unless otherwise agreed by the Parties)
before [*] within [*] business days after the selection of [*]. If the Parties
cannot agree upon [*] within [*] calendar days after the Receiving Party
receives the [*], then the Parties shall retain [*] or its successor, or only if
[*] is not available, some other similar [*], to select the [*] and the Parties
shall participate in a [*] business days in length unless otherwise agreed by
the Parties) before such [*] within [*] business days after the selection of
such [*]. The Parties agree to participate in such [*]. The costs of such [*]
service shall be borne [*]. No judge [*] of a court with jurisdiction over
either Party, or over any such dispute, shall be eligible to serve as a [*]. Any
such [*] shall be conducted in the County of San Diego unless otherwise agreed
by the Parties. All offers, promises, conduct and statements, whether oral or
written, made in the course of the [*] by any of the Parties, their agents,
employees, experts and attorneys, and by the [*], are confidential, privileged
and inadmissible for any purpose, including impeachment, in any litigation or
other proceeding involving the Parties, provided that evidence that is otherwise
admissible or discoverable shall not be rendered inadmissible or
non-discoverable as a result of its use in the [*]. Except as expressly set
forth in this Section 8.11, no conduct of a Party with respect to any such [*]
shall affect any Rights Preserving Excuse or any other right or obligation of a
Party. In particular, no Party's [*] in any [*] shall constitute a breach of any
duty or obligation under this Agreement, or shall create any right, claim or
defense by the other Party.
8.11.4 Cure; Cure Period and Status of Rights Preserving Excuse
During Mediation. In the event that a Rights Preserving Excuse exists in favor
of the Receiving Party and the Receiving Party gives Notice of Rights Preserving
Excuse, then the Obligor Party shall have the Cure Period within which to
effectuate a "Cure" within the meaning of this Section 8.11.4. Until the Cure
Period lapses without the Obligor Party effecting a Cure, or if a court
determines that no Rights Preserving Excuse exists in favor of the Receiving
Party, the Receiving Party must render performance hereunder. "Cure" for
purposes of this Section 8.11 shall mean (a) cure of the breaches set forth in
the Notice of Rights Preserving Excuse by performance by the Obligor Party, to
the extent that the Receiving Party is once again [*] under this Agreement, (b)
performance by the Obligor Party of remedies for such breaches, which remedies
would be deemed adequate under applicable [*] law (including, for example,
payment of monetary damages in certain circumstances), to the extent that the
Receiving Party is once again [*] under this Agreement, or (c) only in the case
of a [*] on the part of the Receiving Party to compel performance of this
Agreement by the Obligor Party, [*] of this Agreement in accordance with
applicable [*]. In the event a Cure is effected by [*] of this Agreement by the
Obligor Party [*], the Receiving Party shall no longer have Rights Preserving
Excuse, but instead will have the rights and remedies accorded to the Receiving
Party under [*] and shall have the ability as set forth therein (among other
things) either to continue to retain certain of its rights under this Agreement
(without creating a Rights Preserving Excuse in favor of the rejecting Obligor
Party), or to treat this Agreement as
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-92-
terminated. In the event that the Obligor Party fails to effect a Cure within
the Cure Period, regardless of whether the Obligor Party resorts to [*] pursuant
to Section 8.11.6 hereof, the Receiving Party shall have the benefit of Rights
Preserving Excuse and [*] in favor of the Obligor Party, and such [*] until such
time as a Cure is effected or a court determines pursuant to Section 8.11.6
hereof that no Rights Preserving Excuse exists in favor of the Receiving Party,
whereupon the Receiving Party shall be required to promptly [*] to the existence
(or assertion) of the Rights Preserving Excuse.
8.11.5 Effect of Failure to Cure. After the end of the Cure Period,
in the absence of Cure, then and only then shall the Receiving Party be [*]
(until Cure is effected or this Agreement is duly terminated in accordance with
Article XIV) from [*] to the Obligor Party (because the Receiving Party failed
to [*]) and the Receiving Party shall retain all of its rights and remedies
against the Obligor Party on account of the failures to perform this Agreement
that gave rise to the Rights Preserving Excuse, and on account of any other
breaches under applicable law, including, if the Receiving Party so elects, to
seek to terminate this Agreement in accordance with Article XIV. In the event
that the Receiving Party shall [*] under the Agreement by reason of a Rights
Preserving Excuse, then until such time as this Agreement is terminated in
accordance with Article XIV, the Obligor Party may obligate the Receiving Party
to [*] owed to the Receiving Party. In the event that (a) the Receiving Party
shall [*] under this Agreement by reason of asserting that Rights Preserving
Excuse exists in its favor, and (b) the Obligor Party [*] that the Receiving
Party has Rights Preserving Excuse and commences [*] pursuant to Section 8.11.6
hereof to resolve such dispute, the Obligor Party may [*] to the Receiving Party
as the Obligor Party contends it is [*] (and which form the basis of Obligor
Party's contention that Receiving Party has not failed to [*] because Obligor
Party is still [*] to prevent Rights Preserving Excuse from arising). Moreover,
the Obligor Party may withhold such performance until the earliest to occur of
the following: (i) a determination by [*] that Rights Preserving Excuse in fact
existed in favor of the Receiving Party, where the Obligor Party [*] as
contemplated by Section 8.11.6 hereof; (ii) a determination by a [*] that Rights
Preserving Excuse in fact existed in favor of the Receiving Party; (iii) a
determination by the [*] that Rights Preserving Excuse did not exist in favor of
the Receiving Party, where the Receiving Party [*] as contemplated by Section
8.11.6 hereof, provided that the Receiving Party [*] on the grounds of Rights
Preserving Excuse; and/or (iv) a determination by a [*] that Rights Preserving
Excuse did not exist in favor of the Receiving Party, provided that the
Receiving Party [*] on the grounds of Rights Preserving Excuse. The Receiving
Party's election [*] hereunder so long as Rights Preserving Excuse exists, shall
not in and of itself [*] the Obligor Party from [*] of its affirmative
obligations under the Agreement, nor shall it give the Obligor Party grounds to
terminate this Agreement under Article XIV.
8.11.6 Legal Proceedings. If the Parties for whatever reason shall
fail to reach agreement on the issues of whether a Rights Preserving Excuse
exists and/or whether the Obligor Party has effected a Cure prior to any
termination of this Agreement pursuant to Article XIV, then either Party may
initiate a legal proceeding to resolve such dispute(s). The Parties hereby [*]
consent to the [*] jurisdiction of the courts of [*] for any action, suit or
proceeding arising out of or relating to any such dispute, and agree not to
commence any action, suit or proceeding related thereto except in such courts.
The Parties further hereby [*] consent to, and [*] to, the laying of venue of
any action, suit or proceeding (other than appeals therefrom) arising out of or
relating to this Agreement in [*], and hereby further [*] waive and agree not to
plead or claim in any such court that any such action, suit or proceeding
brought in any such court has been brought [*]. Any Party that fails to [*]
hereunder such that a Rights Preserving Excuse arises in favor of the other
Party shall be liable for [*] to such other Party as well as being subject to
[*], and any Party that fails to [*] hereunder on the ground that a Rights
Preserving Excuse exists in its favor shall be liable for [*] to the other Party
in the event that a court determines that there was no such Rights Preserving
Excuse as well as being subject to [*]. The Parties also agree to [*] their
respective rights to a [*] of any claim or cause of action based upon or arising
out of any of this Agreement or any of the transactions contemplated herein,
including contract claims, tort claims, breach of duty claims, and all
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-93-
other common law or statutory claims. The Parties represent that each has
reviewed [*] following consultation with legal counsel. In the event of
litigation, a copy of this agreement may be filed as a written consent to a
trial [*].
8.12 No Other Rights. For the avoidance of doubt, Medarex shall have no
right, express or implied, with respect to the Kirin Technology, Kirin
Trademarks, or any of Kirin's right, title and interest in the KM Patent Rights,
KM Know-How, or KM Trademarks, and Kirin shall have no right, express or
implied, with respect to the Medarex Technology, Medarex Trademarks, or any of
Medarex's right, title and interest in the KM Patent Rights, KM Know-How, or KM
Trademarks, in each case except as expressly provided in this Agreement.
ARTICLE IX
TRANSFER OF MATERIALS
9.1 Transfer of Mice and Mice Materials.
9.1.1 Transfer of Materials Between the Parties.
(a) Transfer of Medarex Materials and Kirin Materials Between
the Parties. From time to time upon written reasonable request by Kirin, Medarex
shall provide Medarex Materials to Kirin (or its Affiliates) for use by Kirin or
Kirin Affiliates in connection with the research, development, and
commercialization of Collaboration Products, in such quantities as are
reasonably available. From time to time upon written reasonable request by
Medarex, Kirin shall provide Kirin Materials to Medarex (or its Affiliates) for
use by Medarex and Medarex Affiliates in connection with the research,
development, and commercialization of Collaboration Products, in such quantities
as are reasonably available. Subject to Section 9.1.2 hereof, each Party also
agrees to provide Materials in such quantities as are reasonably available and
as the other Party may from time to time reasonably request [*] in connection
with the research, development, and commercialization of Collaboration Products.
(b) Further Agreement Governing Transfer of Materials.
Promptly following the Effective Date, the Parties shall cooperate in good faith
to agree on a process for the transfer of Materials from one Party to the other
Party pursuant to Section 9.1.1(a) and to agree upon any additional terms that
shall apply to such transfer(s), and shall to the extent determined to be
reasonably necessary by the Parties, negotiate in good faith to enter into a
separate written agreement governing such transfer(s), the terms of which shall
include, without limitation, [*] for the provision of services relating to the
transfer of Materials.
(c) No Sale, Lease or Transfer of Title. Notwithstanding
anything contained in this Agreement, no transfer by a Party to the other Party
of any Collaboration Mice or any Mice Materials relating thereto, whether
pursuant to this or any other agreement between the Parties that may be entered
into pursuant to Section 9.1.1(b) hereof, or that may have been entered into
prior to the Effective Date, shall constitute a sale, offer for sale, lease,
offer for lease or other transfer of title to any such Collaboration Mice or
Mice Materials derived therefrom.
9.1.2 Transfer of Collaboration Mice and Mice Materials to Third
Parties and Control Requirements Relating Thereto.
(a) Transfer of Collaboration Mice and Mice Materials. The
Parties agree that from and after the Effective Date each Party and its
Affiliates shall be permitted, in connection with
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-94-
the exercise of licenses granted to such Party in Article VIII hereof, and
subject to the terms and conditions of this Agreement, to transfer Collaboration
Mice and/or Mice Materials derived therefrom to such Party's Affiliates and
Third Parties that have been granted rights to use such Collaboration Mice in
accordance with Article VIII hereof, for use by such Affiliates and Third
Parties in the research, development, and/or commercialization of Collaboration
Products; provided, however, that prior to making any such transfer of
Collaboration Mice or Mice Materials derived therefrom to an Affiliate or a
Third Party, such Party shall have entered into a written agreement with such
Affiliate or Third Party that governs the terms of such transfer, which terms
shall be consistent with the terms and conditions of this Agreement, and, in the
case of a Third Party, shall prohibit such Third Party from further transferring
such mice except to the extent expressly permitted in Section 8.5.1 and 8.5.2
hereof, as applicable; provided, further, that Kirin shall not transfer,
directly or indirectly, any Medarex Mice to any Affiliate or Third Party, and
Medarex shall not transfer, directly or indirectly, any Kirin Mice to any
Affiliate or Third Party, without the prior written consent of the other Party.
For the avoidance of doubt, Medarex (and its Affiliates) may provide Medarex
Mice and/or KM-Mice directly to Medarex Affiliates and Third Parties, and Kirin
(and its Affiliates) may provide Kirin Mice and KM-Mice directly to Kirin
Affiliates and Third Parties in connection with the research, development, and
commercialization of Collaboration Products, on terms and conditions consistent
with this Agreement. Notwithstanding anything contained in this Agreement,
neither Party shall be permitted, in the exercise of its rights hereunder, to
sell, offer to sell, lease, offer to lease or otherwise transfer title to any
Collaboration Mice or Mice Materials derived therefrom. For the avoidance of
doubt, nothing contained in this Section 9.1.2(a) shall apply to, or otherwise
restrict, transfers of Medarex Mice by Medarex to an Affiliate or Third Party.
(b) Control Requirements. Each Party hereby acknowledges the
value of the Collaboration Mice and Mice Materials derived therefrom, as well as
the possibility that, absent implementation and compliance with reasonable
security precautions, the Collaboration Mice and Mice Materials derived
therefrom may be transferred to or acquired by, legally or otherwise, Third
Parties not in privity with either Party, their respective Affiliates, or
sublicensees. Accordingly, each Party agrees, and shall cause its Affiliates and
sublicensees with which it enters into agreements after the Effective Date to
agree, to implement and maintain such procedures as it, in its reasonable
discretion, believes to be necessary or useful to prevent unauthorized access to
or transfer of Collaboration Mice and/or Mice Materials derived therefrom to
unauthorized Third Parties. Upon written request, from time to time during the
Term hereof but in no event more often than once every two (2) years, each Party
agrees to provide the other Party with a detailed written summary of the
procedures it implements and maintains in this regard.
9.1.3 Transfer and Use of Collaboration Antibodies and Antibody
Materials to Third Parties. The Parties agree that each Party and its respective
Affiliates shall be permitted, subject to the terms and conditions of this
Agreement, to transfer Collaboration Antibodies (and Antibody Materials related
thereto) to Affiliates and Third Parties for research, analysis,
characterization, evaluation, clinical trials and commercialization, with regard
to such transferred Collaboration Antibodies (and Antibody Materials related
thereto), and that, subject to the terms and conditions of this Agreement, the
transferring Party may permit such Affiliates and Third Parties to further
transfer such Collaboration Antibodies (and Antibody Materials related thereto)
to other Affiliates or Third Parties (a) to perform evaluation, clinical trials,
research, and development activities with respect to such Collaboration
Antibodies, and (b) subject to Section 8.5.1(d) hereof, to commercialize such
Collaboration Antibodies and Antibody Products containing such Collaboration
Antibodies (and Antibody Materials relating thereto).
9.2 Information Disclosure. Except to the extent prohibited by the terms
of an agreement with a Third Party or covered by the attorney-client privilege,
in response to a reasonable request by the other
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-95-
Party, each Party shall, without additional compensation, disclose and make
available to such other Party, information, data, and documentation that is
created or otherwise obtained during the Term of this Agreement, which relates
to the non-requesting Party's Patent Rights and Know-How, or KM Patent Rights
and KM Know-How, and is conceived, reduced to practice, or otherwise developed
solely by its (or any of its Affiliates') employees and/or agents, or jointly by
one or more of its (or any of its Affiliates') employees and/or agents and one
or more employees and/or agents of the other Party (or its Affiliates), solely
for the receiving Party's use within the scope of this Agreement, but excluding
Information and Inventions (a) that would fall within the definition of
"Excluded Claims" or "Excluded Know-How"; or (b) relating to such Party's (or
any of its Affiliates', licensees' or sublicensees') Production Process
Technology.
9.3 Production Process Technology. Notwithstanding anything to the contrary
in this Article IX or elsewhere in this Agreement, neither Party shall be
obligated to disclose or provide any of its Production Process Technology to the
other Party or any of its sublicensees except as may be required or permitted
under a separate written agreement, if any, entered into by the Parties.
ARTICLE X
LICENSE FEES, MILESTONES, ROYALTIES AND OTHER PAYMENTS
10.1 Initial License Fees and Other Consideration.
10.1.1 Initial License Fee. Medarex hereby acknowledges that Kirin
has paid to Medarex a non-refundable, non-creditable Initial Fee.
10.1.2 Other License Consideration. Subject to the terms and
conditions set forth in this Agreement, until the earlier to occur of (a) [*]
and (b) the date of the payment of monies by Kirin to Medarex that causes the
total amount paid by Kirin to Medarex pursuant to this Article X (including the
Initial Fee as set forth in Section 10.1.1 hereof and payments made by Kirin to
Medarex pursuant to this Section 10.1.2) to equal or exceed [*] Kirin shall (i)
pay to Medarex the amount that equals [*] of the sum of (A) the balance of the
aggregate Partner Royalties paid to Kirin by its Partners with respect to Kirin
Partner Projects, after deduction of amounts paid to Medarex pursuant to Section
10.7.1 hereof, and (B) the balance of the aggregate Partner Payments paid to
Kirin by its Partners with respect to Kirin Partner Projects, after deduction of
the amounts paid to Medarex pursuant to Section 10.7.3 hereof, and (C) the
balance of the aggregate payments paid to Kirin by Kirin Partners with respect
to Kirin Partner Projects as reservation license fees, commercial license fees,
Antigen exclusivity fees, or the equivalent of any of the foregoing, after
deduction of the amounts paid to Medarex pursuant to Sections 10.2.2, 10.3.1(b)
and 10.4.2 hereof, as applicable; and (ii) pay to Medarex the amount that equals
[*] of the sum of (A) the amounts actually paid to Kirin by Medarex pursuant to
Section 10.7.2 hereof, (B) the amounts actually paid to Kirin by Medarex
pursuant to Section 10.7.4 hereof, including, without limitation, any Partner
Payments received by Medarex from Special Licensees, (C) the amounts actually
paid to Kirin by Medarex pursuant to Sections 10.2.2, 10.3.1(b) and 10.4.2
hereof, and (D) the amounts actually paid to Kirin by Medarex pursuant to
Section 10.7.5(c)(i) and 10.7.5(c)(ii) hereof.
10.1.3 Collaboration Products in Development [*]. In the event that
Kirin, pursuant to Section 10.7.5(c)(i) hereof, [*] with respect to any
Collaboration Product developed in connection with a Kirin Partner Project, and
any such Collaboration Product has achieved [*] described in [*] of the [*]
hereof (each, a [*]) as of [*], then by [*], Kirin shall (a) provide to Medarex
notice of the [*] with respect to each such Collaboration Product, and (b) make
a payment to Medarex in the amount (the "Reconciliation Amount"), if any, by
which X exceeds Y, whereby: (i) X is the [*] that [*] pursuant to
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-96-
[*] hereof through [*], had [*] with respect to such Collaboration Product been
[*] the [*] to the [*] (as defined in this Section 10.1.3); and (ii) Y is the
[*] pursuant to [*] hereof through [*], on account of [*] the [*] with respect
to such Collaboration Product; provided, however, that for purposes of
determining the amount owed by Kirin to Medarex pursuant to this Section 10.1.3
in connection with a Kirin Partner Project with a [*] with respect to a
Collaboration Product, the Reconciliation Amount shall equal [*] of the [*]
calculated for each [*], between (A) the [*] of (1) the amount that [*] pursuant
to [*] hereof had the [*] for such [*] equaled the [*] for such [*], and (2) a
fraction, the denominator of which is the Kirin Designated Exchange Rate for
purchase of United States Dollars with [*] in effect on the date provided in
Section 10.14 hereof for identifying the applicable Kirin Designated Exchange
Rate with respect to such Partner Payment (or if no Partner Payment is made with
respect to such [*], the Kirin Designated Exchange Rate in effect on the [*] day
after the end of the Calendar Quarter in which such [*] occurs), and the
numerator of which is the Kirin Designated Exchange Rate for purchase of United
States Dollars with [*] in effect on the [*], and (B) the amount of the payment
[*] pursuant to [*] hereof with respect to the [*], if any, related to such [*].
Payment of such amount by Kirin shall not affect Kirin's obligation to make
payments to Medarex with respect to such Collaboration Product pursuant to
Section 10.7.5(c)(i) or 10.7.5(c)(ii) hereof, as applicable. For purposes of
this Section 10.1.3, [*] Notwithstanding the foregoing, however, in no event
shall the total aggregate payment by Kirin to Medarex pursuant to Section 10.1.2
hereof and this Section 10.1.3 [*].
10.2 Reservation License Fees. In the event that a Party exercises its
rights under a Reservation License with respect to an Antigen pursuant to
Section 4.2.2 or 4.2.3 hereof, as applicable, then such Party shall pay to the
other Party a Reservation License Fee, subject to the terms and conditions of
this Agreement.
10.2.1 In-House Projects. If a Party exercises its rights under a
Reservation License in connection with an In-House Project, such Party shall be
permitted to exercise rights under such Reservation License with respect to a
particular Reservation Target during the [*] Reservation License Period and
during [*] renewal periods pursuant to Section 4.2.4 hereof (for a total of up
to [*] from the date of first exercise of such License with respect to such
Reservation Target) [*] on account of such exercise. In the event that the Party
exercising rights under such Reservation License renews such License [*] in
accordance with the terms and conditions of Section 4.2.4 hereof, the Party
exercising rights under such Reservation License shall pay to the other Party a
Reservation License Fee in the amount of [*] with respect to the [*] renewal and
an [*] with respect to [*] renewal, if any, which payment obligations shall
accrue at such time as such Party exercises its rights under its Commercial
License with respect to such Target and shall be payable within [*] days after
the date of selection by such Party of such Target as a [*] Target, [*] Target,
or [*] Target pursuant to Article V hereof; provided, however, that if such
Party designates such Target as a [*] Target or an [*] Target pursuant to
Section 5.2 or 5.4 hereof, as applicable, such [*] such Party with respect to
such Target. For the avoidance of doubt, once a Party has exercised a
Reservation License with respect to a particular Reservation Target and enjoyed
the benefit of such exercise during the initial Reservation License Period (and
up to [*] renewal periods) [*] in accordance with the terms of this Section
10.2.1, in the event that such Reservation License terminates or expires and
such Party subsequently exercises its rights under a Reservation License with
respect to the same Reservation Target pursuant to Section 4.2.2 or 4.2.3
hereof, the first Reservation License Period (and [*] renewal periods) and the
[*] Reservation License Period (and [*] renewal periods) with respect to such
Reservation Target shall be aggregated for purposes of determining whether such
Party [*] on account of such exercise (and [*] in the event of renewals).
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-97-
10.2.2 Partner Projects. If a Party exercises its rights under a
Reservation License in connection with a Partner Project, subject to Section
10.1.2 hereof, such Party shall pay to the other Party within [*] days after the
exercise of such Reservation License (a) the [*] of (i) [*], and (ii) the amount
that equals [*] of any amount paid to such Party (or its Affiliate) by its
Partner as a reservation license fee (or its equivalent), with respect to any
sublicense under such Reservation License granted in connection with a Partner
Project involving a Partner Headquartered in such Party's Primary Promotional
Area, calculated in U.S. Dollars on the basis of the Designated Exchange Rate in
effect as of the date of payment pursuant to this Section 10.2.2; and (b) the
[*] of (i) [*], and (ii) the amount that equals [*] of any amount paid to the
Party (or its Affiliate) by its Partner as a reservation license fee (or its
equivalent), with respect to any sublicense under such Reservation License
granted in connection with a Partner Project that includes a Partner
Headquartered in the other Party's Primary Promotional Area, converted into U.S.
Dollars on the basis of the Designated Exchange Rate in effect as of the date of
payment pursuant to this Section 10.2.2; provided, however, that if rights under
a Reservation License are exercised by a Party in connection with a Partner
Project involving a Partner Headquartered in such Party's Primary Promotional
Area, such Party may request that [*] described above[*]; and provided, further
that if rights under a Reservation License are exercised by a Party in
connection with a Partner Project involving a Partner Headquartered in the other
Party's Primary Promotional Area, such Party may request that the [*] described
above, which [*], for the avoidance of doubt, Medarex hereby acknowledges that
it has granted to Kirin [*] in relation to [*] by Kirin to Medarex hereunder
with respect to each such sublicense for the [*] Reservation License Period, and
a [*] renewal of such sublicense); and provided, further, that with respect to
any sublicense under a Reservation License granted by Medarex to a [*] in
connection with a Medarex Partner Project, Medarex shall be obligated to pay
Kirin, in lieu of the amount set forth in clause (a) of this Section 10.2.2, the
amount that [*] of the amount, if any, paid to Medarex (or its Affiliate) by
such [*] as a reservation license fee (or its equivalent); and provided,
further, that with respect to any sublicense under a Reservation License granted
by Kirin to [*], the amount due from Kirin to Medarex pursuant to this Section
shall be an amount equal to the [*] of (x) the amount calculated in clause
(a)(ii) of this Section 10.2.2, and (y) the [*] and a fraction, the denominator
of which is the Kirin Designated Exchange Rate for purchase of United States
Dollars with [*] in effect on [*] for identifying the applicable Kirin
Designated Exchange Rate with respect to such payment, and the numerator of
which is the Kirin Designated Exchange Rate for purchase of United States
Dollars with [*] in effect on the [*]. In the event that a Party exercises a
Reservation License for the purpose of [*] relating to the use of Collaboration
Mice to raise Antibodies to such Antigen(s), the other Party hereby agrees that
in the event that, within [*] days after exercising a Reservation License for
[*], the exercising Party [*] and provides notice thereof to the other Party,
the Reservation License shall terminate as of the date of such notice and the
other Party [*] the exercising Party under this Section 10.2.2 with respect to
such Antigen(s).
10.3 Commercial License Fees. In the event that a Party exercises its
rights under a Commercial License pursuant to Section 4.3 hereof, such Party
shall pay to the other Party a Commercial License Fee in the amount[*] required
pursuant to Section 10.3.1(a) or 10.3.1(b), subject to the terms and conditions
of this Agreement.
10.3.1 Commercial License for In-House Projects and Partner Projects.
(a) In-House Projects. If a Party exercises its rights under a
Commercial License in connection with an In-House Project for an Antigen that is
designated as a [*] Target, such Party shall pay to the other Party a [*]
Commercial License Fee in the amount of [*] within [*] days after the date of
designation of such Target as a [*] Target pursuant to Article V hereof. If a
Party exercises its rights under a Commercial License in connection with an
In-House Project for an Antigen that is designated as a [*] Target, such Party
shall [*] with respect to such Commercial License.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-98-
(b) Partner Projects. If a Party exercises its rights under a
Commercial License in connection with a Partner Project, subject to Section
10.1.2 hereof, such Party shall pay to the other Party within [*] days after the
exercise thereof (i) the [*] of (A) [*] and (B) the amount that equals [*] of
the amount (which amount shall be [*] for purposes of this calculation by the
amount of any [*] with respect to such License) paid to such Party (or its
Affiliate) by its Partner as a commercial license fee (or its equivalent), with
respect to any sublicense under such Commercial License granted in connection
with a Partner Project involving a Partner Headquartered in such Party's Primary
Promotional Area, converted into U.S. Dollars on the basis of the Designated
Exchange Rate in effect as of the date of payment pursuant to this Section
10.3.1(b); and (ii) the [*] of (A) [*], and (B) the amount that equals [*] of
the amount (which amount shall be [*] for purposes of this calculation by the
amount of any [*] with respect to such License) paid to such Party by its
Partner as a commercial license fee (or its equivalent), with respect to any
sublicense under such Commercial License granted in connection with a Partner
Project involving a Partner Headquartered in the other Party's Primary
Promotional Area, calculated in U.S. Dollars on the basis of the Designated
Exchange Rate in effect on date of payment pursuant to this Section 10.3.1(b);
provided, however, that with respect to any sublicense under a Commercial
License granted by Medarex to [*] in connection with a Medarex Partner Project,
Medarex shall be obligated to pay Kirin, in lieu of the amount set forth in
clause (i) of this Section 10.3.1(b) hereof, the amount that [*] the amount[*]
paid to Medarex by such [*] as a commercial license fee (or its equivalent); and
provided, further, that with respect to any sublicense under a Commercial
License granted to [*], the amount due from Kirin to Medarex pursuant to this
Section shall be an amount equal to the [*] of (x) the amount calculated in
clause (i)(B) of this Section 10.3.1(b), and (y) the [*] and a fraction, the
denominator of which is the Kirin Designated Exchange Rate for the purchase of
United States Dollars with [*] in effect on [*] hereof for identifying the
applicable Kirin Designated Exchange Rate with respect to such payment, and the
numerator of which is the Kirin Designated Exchange Rate for purchase of United
States Dollars with [*] in effect [*].
(c) [*] Commercial License Fee on Account of Certain Antibody
Substitutions. In the event that either (i) Medarex or a Medarex Affiliate in
connection with an In-House Project with respect to a [*] Target, or (ii) a
Medarex Partner, during the course of development and/or commercialization of a
particular Collaboration Product against a particular Commercial Target
substitutes for the Collaboration Antibody contained in such Collaboration
Product an Antibody raised against and with affinity for such Commercial Target
using Medarex Mice, Kirin shall, within [*] days after receiving written notice
thereof from Medarex, [*] as a [*] with respect to such Commercial Target
pursuant to [*] hereof, as applicable, [*] pursuant to [*] hereof with respect
to such [*].
(d) [*] on Account of Certain Antibody Substitutions. The Parties
understand and agree that in the event that either (i) Medarex or a Medarex
Affiliate in connection with a HuMAb Project with respect to an Antigen, or (ii)
a Third Party with which Medarex has entered into a written agreement with
respect to such HuMAb Project, during the course of development and/or
commercialization of a particular Antibody Product containing an Antibody raised
against and with affinity for such Antigen using Medarex Mice, [*] for [*]
raised against and with affinity for such Antigen such that Medarex exercises
rights or grants a sublicense under a Commercial License in accordance with this
Agreement and [*], as applicable, Medarex shall pay to Kirin the amounts payable
pursuant to Section 10.3.1(a) or 10.3.1(b) hereof, as applicable, in connection
with such Commercial License. It is further understood and agreed by the Parties
hereto that in the case of clause (ii) above and where such [*] described above,
even if Medarex [*] when the Commercial License is sublicensed to such Third
Party, Medarex shall [*] to [*] under Section [*] hereof by treating the [*],
for the [*] granted by [*] with respect to the Antibody obtained using [*] Mice
(and for which the [*] was [*]) as an amount [*] a [*] (or
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-99-
its Affiliate) as a [*] (or its equivalent) with respect to a sublicense under
the Commercial License for purposes of Section 10.3.1(b) hereof.
10.4 Antigen Exclusivity Fees. In the event that a Party exercises its
rights under an Antigen Exclusive Commercial License or an Antigen
Semi-Exclusive Commercial License pursuant to Section 4.3 hereof, such party
shall pay to the other Party an Antigen Exclusivity Fee (which amount shall be
in addition to any amount owed by such Party to the other Party pursuant to
Section 10.3 hereof), as follows.
10.4.1 In-House Project. If a Party exercises its rights under an
Antigen Exclusive Commercial License or an Antigen Semi-Exclusive Commercial
License in connection with an In-House Project, such Party shall pay to the
other Party a [*] Antigen Exclusivity Fee in the amount of [*] for each Antigen
with respect to which exclusive rights are exercised by such Party, within [*]
days after the exercise thereof.
10.4.2 Partner Project. If a Party exercises its rights under an
Antigen Exclusive Commercial License or an Antigen Semi-Exclusive Commercial
License in connection with a Partner Project, subject to Section 10.1.2 hereof,
such Party shall pay to the other Party within [*] days after the exercise
thereof [*] Antigen Exclusivity Fee in the amount of [*] for each Antigen with
respect to which exclusive rights are exercised by such Party (on behalf of any
of its Partners) in connection with a Partner Project; provided, however, that
[*] Party [*] to the [*] Party the fee described in this Section 10.4.2 with
respect to any Antigen selected on an exclusive basis (as confirmed by
information in the applicable Antigen List) by or on behalf of a [*] pursuant to
the applicable [*] governing a [*], which agreement was [*] the Effective Date
and [*], and giving [*] to any [*] thereto [*] to the extent that such [*] the
[*] that the Partner is entitled to [*] under the applicable [*] governing the
applicable [*] (with respect to which [*] the Party that enters into such [*]
shall [*] to the other Party an Antigen Exclusivity Fee pursuant to this Section
10.4.2); and provided, further, that [*] Party [*] to the [*] Party the fee
described in this Section with respect to any Antigen selected on an exclusive
basis (as confirmed by the information in the applicable Antigen List) by or on
behalf of a [*] pursuant to a [*] even in the event that the [*] and such [*]
such [*] or [*] a [*] to [*] the [*] with [*] with respect to such Antigen.
10.4.3 Medarex Internal Projects and Other Third-Party Agreements. In
the event that Medarex reserves for itself the right to develop and
commercialize any and all Antibodies and Antibody Products against a particular
Antigen on an exclusive basis in connection with a Medarex Internal Project, as
confirmed by the information on the Shared Exclusive Antigen List, or if Medarex
grants to a Third Party rights under the Medarex Technology (but not the Kirin
Technology or KM Patent Rights) to develop and commercialize any and all
Antibodies and Antibody Products against a particular Antigen on an exclusive
basis, including, without limitation, in connection with a HuMAb License Project
or HuMAb Collaboration Project, as confirmed by the information on the Shared
Exclusive Antigen List or the Medarex Restricted Antigen List, then with respect
to each such Antigen, Medarex shall pay to Kirin a [*] Antigen Exclusivity Fee
in the amount of [*] that Medarex [*] to Kirin [*] described in this Section
with respect to any Antigen selected on an exclusive basis (as confirmed by the
information in the applicable Antigen List) by or on behalf of any [*] pursuant
to a [*] governing a [*], which agreement [*] with [*] and [*], and giving [*]
to any [*] thereto [*] to the extent that such [*] the [*] that the [*] is
entitled to [*] under the applicable [*] governing the applicable [*] (with
respect to which [*] Medarex shall [*] to Kirin an Antigen Exclusivity Fee
pursuant to this Section 10.4.3); and [*] to Kirin [*] described in this Section
with respect to any Antigen selected on an exclusive basis (as confirmed by the
information in the applicable Antigen List) by or on behalf of a [*] pursuant to
a [*] governing a [*] and is [*] even in the event that [*] and such [*] such
[*] or [*] a [*] to [*] with respect to such Antigen.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-100-
10.5 [*] Target Milestone Payments. Subject to the terms and conditions
set forth in this Agreement, each Party shall make the following payments to the
other Party with respect to each [*] Target selected pursuant to Article V
hereof:
10.5.1 Milestone Payments. Subject to the terms and conditions of
Sections 10.5.2, 10.5.3, 10.5.4, 10.5.5, 10.5.6 and 10.5.7 hereof, the
Designating Party shall make [*] to the other Party, within [*] days following
achievement of the corresponding milestone event, as provided below:
(a) In vivo Therapeutics.
(i) Milestone Payments Owed by Kirin to Medarex. On
a [*] Target Product-by-[*] Target Product basis, if Kirin is the Designating
Party, with respect to each [*] Target Product intended for an In Vivo
Therapeutic Application, Kirin shall make the following milestone payments to
Medarex:
Milestone Event [*] Target Product * [*] Target Product * [*] Target Product*
[*] [*] [*] [*]
* [*] Target Product intended for In Vivo Therapeutic Application
(ii) Milestone Payments Owed by Medarex to Kirin. On
a [*] Target Product-by-[*] Target Product basis, if Medarex is the Designating
Party, with respect to each [*] Target Product intended for an In Vivo
Therapeutic Application, Medarex shall make the following milestone payments to
Kirin:
Milestone Event [*] Target Product * [*] Target Product * [*] Target Product*
[*] [*] [*] [*]
* [*] Target Product intended for In Vivo Therapeutic Application
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-101-
(iii) Order of Payment. For clarification purposes,
the milestone amount payable with respect to any milestone event listed in
Section 10.5.1(a)(i) or 10.5.1(a)(ii) hereof that is achieved by a Party [*]
pursuant to this Agreement shall be the amount listed for the [*], even if such
product was [*] of such Party to achieve [*] pursuant to this Agreement. By way
of example and without limitation, if Kirin is the Designating Party and its [*]
has achieved [*], and its [*] receives [*] would be payable to Medarex with
respect to [*]. If the [*] subsequently receives [*] would be payable to
Medarex.
(b) Ex vivo Therapeutics.
(i) Milestone Payments Owed by Kirin to Medarex.
On a [*] Target Product-by-[*] Target Product basis, if Kirin is the Designating
Party, with respect to each [*] Target Product intended for an Ex Vivo
Therapeutic Application, Kirin shall make the following milestone payments to
Medarex:
Milestone Event [*] Target Product*
[*] [*]
* [*] Target Product intended for Ex Vivo Therapeutic Application
(ii) Milestone Payments Owed by Medarex to Kirin.
On a [*] Target Product-by-[*] Target Product basis, if Medarex is the
Designating Party, with respect to each [*] Target Product intended for an Ex
Vivo Therapeutic Application against such [*] Target, Medarex shall make the
following milestone payments to Kirin:
Milestone Event [*] Target Product*
[*] [*]
* [*] Target Product intended for Ex Vivo Therapeutic Application
(c) Diagnostic Products.
(i) Milestone Payments Owed by Kirin to Medarex.
On a [*] Target Product-by-[*] Target Product basis, if Kirin is the Designating
Party, with respect to each [*]
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-102-
Target Product intended for use in a Diagnostic Application, Kirin shall pay to
Medarex the following [*]; provided, however, that such [*] shall be due [*].
(ii) Milestone Payments Owed by Medarex to Kirin. On a
[*] Target Product-by-[*] Target Product basis, if Medarex is the Designating
Party, with respect to each [*] Target Product intended for use in a Diagnostic
Application, Medarex shall pay to Kirin the following [*]; provided, however,
that such [*] shall be due [*].
(d) Reagent Products.
(i) Milestone Payments Owed by Kirin to Medarex. On a
[*] Target Product-by-[*] Target Product basis, if Kirin is the Designating
Party, with respect to each [*] Target Product intended for use as a Reagent,
Kirin shall pay to Medarex the following [*]; provided, however, that such [*]
shall be due [*].
(ii) Milestone Payments Owed by Medarex to Kirin. On a
[*] Target Product-by-[*] Target Product basis, if Medarex is the Designating
Party, with respect to each [*] Target Product intended for use as a Reagent,
Medarex shall pay to Kirin the following [*]; provided, however, that such [*]
shall be due [*].
10.5.2 Milestone Payments for Multiple [*] Target Products Containing
the Same Collaboration Antibody. Subject to Section 10.5.3 hereof, it is
understood and agreed that, in the event that a Party (or its Affiliate(s)), in
connection with one or more In-House Projects, develops more than one
Collaboration Product containing the same Collaboration Antibody as an active
ingredient and such products are intended for the same type of product
application (i.e., In Vivo Therapeutic Application, Ex Vivo Therapeutic
Application, Diagnostic Application, or Reagent), for purposes of calculating
any milestone payments owed by such Party to the other Party pursuant to
Sections 10.5.1(a), 10.5.1(b), 10.5.1(c), and 10.5.1(d) hereof, as applicable,
with respect to any such Collaboration Products that are designated as [*]
Target Products, all such Collaboration Products shall be deemed to be one and
the same Collaboration Product. If all such Collaboration Products are
designated as [*] Target Products, the milestone payment for each milestone
event shall be owed by the Party developing and commercializing such [*] Target
Products [*] and shall be triggered at the time that the [*]. By way of example
and without limitation, if Medarex [*] with respect to a [*] Target Product
intended for an In Vivo Therapeutic Application, and such [*] Target Product
contains a Collaboration Antibody, designated as Collaboration Antibody A for
purposes of this example, Medarex shall make a milestone payment to Kirin
pursuant to Section 10.5.1(a)(ii) totaling [*]. If Medarex [*] with respect to a
[*] Target Product intended for an In Vivo Therapeutic Application and such [*]
Target Product also contains Collaboration Antibody A, Medarex [*] a milestone
payment to Kirin on account of the [*].
10.5.3 Milestone Payments for [*] Target Product Containing More than
One Collaboration Antibody. It is understood and agreed that, in the event that
a [*] Target Product contains more than one Collaboration Antibody, for purposes
of calculating any milestone payments owed by a Party to the other Party with
respect to such [*] Target Product pursuant to Sections 10.5.1(a), 10.5.1(b),
10.5.1(c), and 10.5.1(d) hereof, as applicable, [*] milestone payments shall be
owed by such Party for [*] as if each such Collaboration Antibody [*]; provided,
however, that neither Party shall be required to treat any such Collaboration
Antibody as [*] subject to [*] milestone payments if such Party, [*] has with
respect to such [*] Target Product containing such Collaboration Antibody (a)
paid to the other Party a milestone payment on account of [*] pursuant to
Section 10.5.1(a), 10.5.1(b), 10.5.1(c), or 10.5.1(d) hereof, as the case may
be, with respect to [*] containing such Collaboration Antibody, or (b) [*] with
respect to a [*] Target Product containing such Collaboration Antibody. By way
of example and without
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-103-
limitation, if Medarex [*] with respect to a [*] Target Product intended for an
In Vivo Therapeutic Application, and such [*] Target Product contains [*] for
purposes of this example, Medarex shall make milestone payments to Kirin
pursuant to Section 10.5.1(a)(ii) totaling [*]. If, on the other hand, Medarex
[*] with respect to a [*] Target Product intended for an In Vivo Therapeutic
Application, and such [*] Target Product contains [*] for purposes of this
example, and Medarex makes a corresponding milestone payment to Kirin pursuant
to Section 10.5.1(a)(ii) hereof totaling [*], and Medarex [*] with respect to a
[*] intended for an In Vivo Therapeutic Application, and such [*] Target Product
contained [*], Medarex shall make a milestone payment to Kirin pursuant to
Section 10.5.1(a)(ii) hereof totaling [*] for such [*] Target Product.
10.5.4 Milestone Payments for [*] Target Product Containing a [*]
Antibody.
(a) It is understood and agreed that, in the event that a \
Party, during the course of development and/or commercialization of a particular
[*] Target Product against a particular [*] Target and in accordance with the
terms and conditions of Article IV hereof, [*], for purposes of calculating
milestone payments owed by such Party to the other Party with respect to such
[*] Target Products pursuant to Sections 10.5.1(a), 10.5.1(b), 10.5.1(c), and
10.5.1(d) hereof, as applicable, the [*] such that milestone payments that
accrue and are paid prior to the [*] shall be deemed to have been paid with
respect to the [*] and milestone payments triggered upon the occurrence of [*]
shall be payable [*] Target Product. By way of example and without limitation,
if Medarex [*] with respect to a [*] Target Product intended for an In Vivo
Therapeutic Application and pays to Kirin a milestone payment in the amount of
[*] pursuant to Section 10.5.1(a)(ii) hereof, and thereafter Medarex [*],
Medarex [*] but [*] with respect to such [*] Target Product.
(b) It is understood and agreed that, in the event that
Medarex (or any of its Affiliates), during the course of development and/or
commercialization of a particular [*] Target Product against a particular [*]
Target, [*] the Collaboration Antibody contained in such [*] Target Product an
Antibody raised against and with affinity for such [*] Target using Medarex
Mice, from and after the date on which Medarex provides written notice thereof
to Kirin, [*]. In addition, in the event that Medarex, [*], paid to Kirin a
milestone payment(s) on account of Medarex's achieving a milestone event(s) with
respect to the Collaboration Product pursuant to Section 10.5.1(a)(ii),
10.5.1(b)(ii), 10.5.1(c)(ii) or 10.5.1(d)(ii) hereof, upon Medarex's [*] with
respect to [*] Antibody Product, Medarex shall provide notice thereof to Kirin
and Kirin shall within [*] days of the receipt of such notice [*] the amount of
the corresponding milestone payment, [*] pursuant to Section 10.1.2 hereof with
respect to such milestone payment. For the avoidance of doubt, in the event that
Medarex fails or otherwise does not achieve with respect to [*] Antibody Product
[*] for the related Collaboration Product, Kirin [*] the corresponding milestone
payment.
(c) For the avoidance of doubt, it is further understood and
agreed that, in the event that Medarex (or any of its Affiliates), during the
course of development and/or commercialization of a particular Antibody Product
containing an Antibody raised against and with affinity for a particular Antigen
using Medarex Mice in connection with a HuMAb Project, [*] for such Antibody a
Collaboration Antibody raised against and with affinity for such Antigen using
Kirin Mice and/or KM-Mice, and [*], Medarex shall, subject to Article V hereof,
designate the Antigen as a [*] Target or a [*] Target and provide written notice
thereof to Kirin. In the event that Medarex designates such Antigen as a [*]
Target and with respect to any [*] Target Product containing such substituted
Collaboration Antibody achieves one or more of the milestone events set forth in
Sections 10.5.1(a)(ii), 10.5.1(b)(ii), 10.5.1(c)(ii) or 10.5.1(d)(ii) hereof, as
applicable, Medarex shall [*].
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-104-
10.5.5 Milestone Payments for [*] Target Product with Multiple
Indications. It is understood and agreed that, in the event that a [*] Target
Product is developed and/or approved for multiple indications within the same
application (e.g., In Vivo Therapeutic Application), any milestone payments with
respect to such [*] Target Product are payable [*] provided under Section
10.5.1(a), 10.5.1(b), 10.5.1(c), and 10.5.1(d) hereof, as applicable, upon the
[*] with respect to the [*] Target Product.
10.5.6 Milestone Payments for a [*] Target Product with Multiple
Applications.
(a) In the event that a [*] Target Product is developed for
both an In Vivo Therapeutic Application(s) and an Ex Vivo Therapeutic
Application(s), (i) any [*] paid pursuant to Section 10.5.1(a) hereof, with
respect to such [*] Target Product in connection with the achievement of a
milestone event in an In Vivo Therapeutic Application [*] that becomes payable
with respect to the achievement of the same milestone event in an Ex Vivo
Therapeutic Application with respect to such [*] Target Product, and (ii) any
prior milestone payment paid pursuant to Section 10.5.1(b) hereof, with respect
to such [*] Target Product in connection with the achievement of a milestone
event in an Ex Vivo Therapeutic Application [*] that becomes payable with
respect to the achievement of the same milestone event in an In Vivo Therapeutic
Application with respect to such [*] Target Product.
(b) In the event that a [*] Target Product is developed for
either an In Vivo Therapeutic Application(s) or an Ex Vivo Therapeutic
Application(s), (i) any prior milestone payment paid pursuant to Section
10.5.1(a) or 10.5.1(b) hereof, with respect to such [*] Target Product in
connection with the achievement of a milestone event in an In Vivo Therapeutic
Application or Ex Vivo Therapeutic Application [*] that becomes payable with
respect to the achievement of the same milestone event in a Diagnostic
Application or for use as a Reagent directed at the same indication with respect
to such [*] Target Product pursuant to Section 10.5.1(c) or 10.5.1(d) hereof, as
the case may be; and (ii) any prior milestone payment paid pursuant to Section
10.5.1(c) or 10.5.1(d) hereof, with respect to such [*] Target Product in
connection with the achievement of a milestone event in a Diagnostic Application
or for use as a Reagent shall be [*] that becomes payable with respect to the
achievement of the same milestone event in either an In Vivo Therapeutic
Application or an Ex Vivo Therapeutic Application directed at the same
indication with respect to such [*] Target Product pursuant to Section 10.5.1(a)
or 10.5.1(b) hereof, as the case may be. For purposes of this Section 10.5.6(b),
the amounts payable under Sections 10.5.1(c) and 10.5.1(d) hereof shall be
deemed to be milestone payments in respect of [*]. By way of example and without
limitation, if a [*] Target Product is [*] as a product intended for a
Diagnostic Application and the [*] under Section 10.5.1(c) hereof have been made
with respect thereto, and the same [*] Target Product subsequently receives a
[*] for an In Vivo Therapeutic Application, the milestone payable under Section
10.5.1(a) hereof shall be [*] under Section 10.5.1(c) hereof for such [*] Target
Product. If the same [*] Target Product subsequently is further sold as a
Reagent, the milestone payable under Section 10.5.1(d) hereof with respect
thereto shall be [*] the amount payable under Section 10.5.1(d) hereof.
10.5.7 [*]. Except as provided under Section 10.5.6 hereof, [*]
payable under this Agreement [*] that may be due to a Party under this
Agreement.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-105-
10.6 In-House Project Royalties. Subject to the terms and conditions set
forth in this Agreement, each Party shall pay to the other Party the following
royalties based on worldwide aggregate Net Sales of each Collaboration Product
resulting from an In-House Project:
10.6.1 [*] Target Royalties.
(a) Royalties Owed by Kirin to Medarex. Kirin shall pay to
Medarex with respect to sales of [*] Target Products arising from [*] Targets
selected by Kirin pursuant to Section 5.2 hereof the sum of (i) [*] of Net Sales
of such Collaboration Products; and, if applicable, (ii) such amount as [*] with
respect to such sales.
(b) Royalties Owed by Medarex to Kirin. Medarex shall pay to
Kirin with respect to sales of [*] Target Products arising from [*] Targets
selected by Medarex pursuant to Section 5.2 hereof [*] of Net Sales of such
Collaboration Products. With respect to such sales, as between Medarex and
Kirin, Medarex shall [*] for the [*] pursuant to [*], including, without
limitation, to [*].
10.6.2 [*] Target Royalties.
(a) Royalties Owed by the Designating Party. The Designating
Party shall pay to the other Party with respect to sales of a [*] Target Product
selected by the Designating Party pursuant to Article V hereof (i) [*] of Net
Sales of each such Collaboration Product sold for an In Vivo Therapeutic
Application in each Calendar Year and [*] of Net Sales of each such
Collaboration Product sold for an In Vivo Therapeutic Application in such
Calendar Year; and (ii) [*] of Net Sales of each such Collaboration Product sold
for an Ex Vivo Therapeutic Application, Diagnostic Application or a Reagent.
(b) [*]
10.7 Partner Royalties and Partner Payments.
10.7.1 Partner Royalties Owed by Kirin to Medarex.
(a) Subject to Section 10.1.2 hereof, Kirin shall pay to
Medarex during the Term of this Agreement royalties on sales in Asia of each
Collaboration Product resulting from a Kirin Partner Project sold for an In Vivo
Therapeutic Application, as follows: [*] of the [*] of (i) the sum of (A) [*] of
net sales (as defined in the relevant Project Agreement governing the relevant
Partner Project) of each such Collaboration Product sold in each Calendar Year,
and (B) [*] of net sales (as defined in the relevant Project Agreement governing
the relevant Partner Project) of each such Collaboration Product sold in each
Calendar Year, and (ii) the amounts [*] for sales of such Collaboration Product
in Asia during the corresponding period, in each case [*] with respect to such
sales (and subject to Section 10.7.1(e) hereof);
(b) Subject to Section 10.1.2 hereof, Kirin shall pay to
Medarex during the Term of this Agreement royalties on sales in Asia of each
Collaboration Product resulting from a Kirin Partner Project sold for an Ex Vivo
Therapeutic Application, Diagnostic Application or as a Reagent, as follows, [*]
of the [*] of (i) the sum of [*] of net sales (as defined in the relevant
Project Agreement governing the relevant Partner Project) of each such
Collaboration Product sold in each Calendar Year, and (ii) the amounts [*] for
sales of such Collaboration Product in Asia during the corresponding period, in
each case [*] with respect to such sales (and subject to Section 10.7.1(e)
hereof);
(c) Subject to Section 10.1.2 hereof, Kirin shall pay to
Medarex during the Term of this Agreement royalties on sales outside Asia of
each Collaboration Product resulting from a Kirin
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-106-
Partner Project sold for an In Vivo Therapeutic Application, as follows: [*] of
the [*] of (i) the sum of (A) [*] of net sales (as defined in the relevant
Project Agreement governing the relevant Partner Project) of each such
Collaboration Product sold in each Calendar Year, and (B) [*] of net sales (as
defined in the relevant Project Agreement governing the relevant Partner
Project) of each such Collaboration Product sold in each Calendar Year, and (ii)
the amounts actually received by Kirin from a Partner for sales of such
Collaboration Product outside Asia during the corresponding period, in each case
[*] with respect to such sales (and subject to Section 10.7.1(e) hereof); and
(d) Subject to Section 10.1.2 hereof, Kirin shall pay to
Medarex during the Term of this Agreement royalties on sales outside Asia of
each Collaboration Product resulting from a Kirin Partner Project sold for an Ex
Vivo Therapeutic Application, Diagnostic Application or as a Reagent, as
follows: [*] of the [*] of (i) [*] of net sales (as defined in the relevant
Project Agreement governing the relevant Partner Project) of each such
Collaboration Product sold in each Calendar Year, and (ii) the amounts [*] for
sales of such Collaboration Product outside Asia during the corresponding
period, in each case [*] with respect to such sales (and subject to Section
10.7.1(e) hereof); and
(e) [*] the amounts payable by Kirin to Medarex pursuant to
Sections 10.7.1(a), (b), (c), and (d) hereof, as applicable, [*] with respect to
sales of each Collaboration Product resulting from a Kirin Partner Project [*]
with respect to such sales.
10.7.2 Partner Royalties Owed by Medarex to Kirin.
(a) Subject to Section 10.1.2 hereof, Medarex shall pay to
Kirin during the Term of this Agreement royalties on sales in Asia of each
Collaboration Product resulting from a Medarex Partner Project sold for an In
Vivo Therapeutic Application, as follows: [*] of the [*] of (i) the sum of (A)
[*] of net sales (as defined in the relevant Project Agreement governing the
relevant Partner Project) of each such Collaboration Product sold in each
Calendar Year, and (B) [*] of net sales (as defined in the relevant Project
Agreement governing the relevant Partner Project) of each such Collaboration
Product sold in each Calendar Year, and (ii) amounts [*] for sales of such
Collaboration Product in Asia during the corresponding period, in each case [*]
with respect to such sales;
(b) Subject to Section 10.1.2 hereof, Medarex shall pay to
Kirin during the Term of this Agreement royalties on sales in Asia of each
Collaboration Product resulting from a Medarex Partner Project sold for an Ex
Vivo Therapeutic Application, Diagnostic Application or as a Reagent, as
follows: [*] of the [*] of (i) [*] of net sales (as defined in the relevant
Project Agreement governing the relevant Partner Project) of each such
Collaboration Product sold in each Calendar Year, and (ii) amounts [*] for sales
of such Collaboration Product in Asia during the corresponding period, in each
case [*] with respect to such sales;
(c) Subject to Section 10.1.2 hereof, Medarex shall pay to
Kirin during the Term of this Agreement royalties on sales outside Asia of each
Collaboration Product resulting from a Medarex Partner Project sold for an In
Vivo Therapeutic Application, as follows: [*] of the [*] of (i) the sum of (A)
[*] of net sales (as defined in the relevant Project Agreement governing the
relevant Partner Project) of each such Collaboration Product sold in each
Calendar Year, and (B) [*] of net sales (as defined in the relevant Project
Agreement governing the relevant Partner Project) of each such Collaboration
Product sold in each Calendar Year, and (ii) the amounts [*] for sales of such
Collaboration Product outside Asia during the corresponding period, in each case
[*] with respect to such sales;
(d) Subject to Section 10.1.2 hereof, Medarex shall pay to
Kirin during the Term of this Agreement royalties on sales outside Asia of each
Collaboration Product resulting from a Medarex
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-107-
Partner Project sold for an Ex Vivo Therapeutic Application, Diagnostic
Application or as a Reagent, as follows: [*] of the [*] of (i) [*] of net sales
(as defined in the relevant Project Agreement governing the relevant Partner
Project) of each such Collaboration Product sold in each Calendar Year, and (ii)
the amounts [*] for sales of such Collaboration Product outside Asia during the
corresponding period, in each case [*].
10.7.3 Partner Payments Owed by Kirin to Medarex. Subject to
Sections 10.1.2 and 10.7.5 hereof, Kirin shall pay to Medarex a share of the
Kirin Partner Payments (other than Partner Payments that constitute a
reservation license fee, commercial license fee or an exclusivity fee, or the
equivalent of any of the foregoing) received by Kirin (and/or its Affiliates),
as follows: (a) [*] of such Partner Payments received during the Term of this
Agreement from Kirin Partners which are Headquartered in Asia; and (b) [*] of
such Partner Payments received during the Term of this Agreement from Kirin
Partners which are Headquartered in countries outside Asia.
10.7.4 Partner Payments Owed by Medarex to Kirin. Subject to
Sections 10.1.2 and 10.7.5 hereof, Medarex shall pay to Kirin a share of the
Medarex Partner Payments (other than Partner Payments that constitute a
reservation license fee, commercial license fee or an exclusivity fee, or the
equivalent of any of the foregoing) received by Medarex (and/or its Affiliates),
as follows: (a) [*] of such Partner Payments received during the Term of this
Agreement from Medarex Partners which are Headquartered in countries outside
Asia; and (b) [*] of such Partner Payments received during the Term of this
Agreement from Medarex Partners which are Headquartered in Asia; provided,
however, that with respect to any such Partner Payments received by Medarex
(and/or its Affiliates) during the Term of this Agreement pursuant to the terms
of any [*], Medarex shall [*] to pay Kirin [*] of such Partner Payments.
10.7.5 [*] Partner Payments. The [*] amounts payable by a Party to
the other Party pursuant to Section 10.7.3 or 10.7.4 hereof, as applicable, on
account of Partner Payments received by such Party (and/or its Affiliates) in
connection with any particular Partner Project with respect to each
Collaboration Product intended for use as an In Vivo Therapeutic Application for
which such Party's Partner has received a sublicense under a Commercial License
shall be as follows:
(a) [*] Partner Payments by Kirin to Medarex. Subject to
Section 10.7.5(c), the [*] Partner Payment payable by Kirin to Medarex on
account of Partner Payments in connection with any particular Partner Project
with respect to any Collaboration Product shall be the greater of (i) the amount
that would be owed by Kirin to Medarex pursuant to Section 10.7.3 hereof (before
making adjustments pursuant to Section 10.1.2 hereof), and (ii) the amount
calculated using the formula [*] in the case of Partner Payments received by
Kirin (and/or its Affiliates) from Kirin Partners which are headquartered in
countries outside Asia, and the amount calculated using the formula [*], in the
case of Partner Payments received by Kirin (and/or its Affiliates) from Kirin
Partners which are headquartered in Asia, where [*].
(b) [*] Partner Payments by Medarex to Kirin. Subject to
Section 10.7.5(c), the [*] Partner Payment payable by Medarex to Kirin on
account of Partner Payments in connection with any particular Partner Project
with respect to any Collaboration Product shall be the greater of (i) the amount
that would be owed by Medarex to Kirin pursuant to Section 10.7.4 hereof (before
making adjustments pursuant to Section 10.1.2 hereof), and (ii) the amount
derived by the formula [*], in the case of Partner Payments received by Medarex
(and/or its Affiliates) from Medarex Partners which are headquartered in Asia,
and the amount derived by the formula [*], in the case of Partner Payments
received by Medarex (and/or its Affiliates) from Medarex Partners which are
headquartered in countries outside Asia, where [*] has the same meaning as set
forth in Section 10.7.5(a) hereof.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-108-
(c) Calculation and Timing of [*] Partner Payments.
(i) In General. Except as provided in Section
10.7.5(c)(ii) hereof, the calculation of [*] Partner Payments shall be [*] with
respect to each Collaboration Product until the receipt by the Paying Party from
the Partner of the last milestone payment provided for by the applicable Project
Agreement with respect to such Collaboration Product. Within [*] days of [*],
the Paying Party shall calculate the [*] Partner Payment and the [*] (as defined
below) for such Collaboration Product and provide notice thereof and support
therefor to the Non-Paying Party. Subject to Section 10.1.2 hereof, the Paying
Party shall, within [*] days after the end of the Calendar Quarter in which [*]
is achieved, make a payment to the Non-Paying Party in the amount (the [*]), if
any, by which the [*] Partner Payment calculated pursuant to Section 10.7.5(a)
or 10.7.5(b) hereof, as applicable, [*] through the end of such Calendar Quarter
by the Paying Party to the Non-Paying Party on account of Partner Payments
relating to such Collaboration Product in accordance with Section 10.7.3 or
10.7.4 hereof, as applicable, which payment for purposes of this Agreement shall
be treated as a payment made pursuant to Section 10.7.3 or 10.7.4 hereof, as
applicable; provided, however, that in the case of [*] as the Paying Party, if
amounts owed by [*] pursuant to Section 10.7.4 hereof with respect to such
Collaboration Product are [*] pursuant to Section 10.1.2 hereof, for purposes of
this Section 10.7.5(c)(i), such [*] pursuant to Section 10.7.4 hereof in
connection with such Collaboration Product, if such [*] pursuant to Section
10.1.2 hereof had not been made; and provided, further, that in the case of the
[*] Partner Payment on account of Partner Payments made to Kirin by a [*] in
connection with a Partner Project with respect to a Collaboration Product, the
[*] shall equal [*] of the [*], calculated for each [*], between (x) the [*],
where [*] is the payment [*], and a fraction, the denominator of which is the
Kirin Designated Exchange Rate for purchase of United States Dollars with [*] in
effect on the date provided in Section 10.14 hereof for identifying the
applicable Kirin Designated Exchange Rate in respect of the Partner Payment, if
any, for [*] the Kirin Designated Exchange Rate in effect on the [*] day after
the end of the Calendar Quarter in which such milestone is achieved), and the
numerator of which is the Kirin Designated Exchange Rate for purchase of United
States Dollars with [*] in effect on the [*], and (y) the amount of the payment
by Kirin to Medarex pursuant to Section 10.7.3 hereof in respect of such Partner
Payment. A sample calculation of the [*] in the case of a Partner Project with a
[*] is attached hereto as Exhibit E. If, after the Paying Party makes a payment
to the Non-Paying Party pursuant to this Section 10.7.5(c)(i), the Paying Party
receives from the Partner that has rights to Exploit such Collaboration Product
additional amounts that would trigger a payment by the Paying Party to the
Non-Paying Party pursuant to Section 10.7.3 or 10.7.4 hereof, as applicable, the
amount paid by the Paying Party to the Non-Paying Party pursuant to this Section
10.7.5(c)(i) shall [*] those [*] amounts that would otherwise be owed by the
Paying Party to the Non-Paying Party pursuant to Section 10.7.3 or 10.7.4
hereof, as applicable.
(ii) Converted or Discontinued Collaboration Products.
In the event that (a) a Partner Project with respect to a Collaboration Product
converts to an In-House Project pursuant to Section 7.2 hereof [*]; (b) a
Partner discontinues development and commercialization of a Collaboration
Product [*]; or (c) the Paying Party has a reasonable basis to believe that such
Partner [*] (each such period a [*]), the Paying Party shall provide to the
other Party notice thereof. In any such event, the Parties shall use the
formulas set forth in Section 10.7.5(a) hereof for purposes of calculating the
[*] Partner Payment with respect to such Collaboration Product, except that, in
lieu of the meaning assigned thereto in Section 10.7.5(a), [*] the conversion of
the Partner Project to an In-House Project, the discontinuation of development
and commercialization of such Collaboration Product or the end of the [*] for
such Collaboration Product, as the case may be. Subject to Section 10.1.2
hereof, the Paying Party shall, within [*] days after the date that marks the
end of the Calendar Quarter in which such conversion of Partner Project to
In-House Project or such discontinuation occurs or the end of the [*] with
respect to such Collaboration Product, as the case may be, make a payment to the
Non-Paying Party in the amount, if any, by which the [*] Partner Payment [*]
paid through the end of such Calendar Quarter by the Paying
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-109-
Party to the Non-Paying Party on account of Partner Payments with respect to
such Collaboration Product in accordance with Section 10.7.3 or 10.7.4 hereof,
as applicable, which payment for purposes of this Agreement shall be treated as
a payment made pursuant to Section 10.7.3 or 10.7.4 hereof, as applicable;
provided, however, that in the case of [*] as the Paying Party, if amounts owed
by [*] pursuant to Section 10.7.4 hereof with respect to such Collaboration
Product are [*] pursuant to Section 10.1.2 hereof or, in the case of a [*],
pursuant to Section 10.7.4 hereof, for purposes of this Section 10.7.5(c)(ii),
such [*] pursuant to Section 10.7.4 hereof in connection with such Collaboration
Product, if such [*] pursuant to Section 10.1.2 hereof had not been made. If,
after the Paying Party makes such payment to the Non-Paying Party pursuant to
this Section 10.7.5(c)(ii), development and commercialization of such
Collaboration Product is resumed by such Partner or the In-House Project into
which the Partner Project had converted converts back to a Partner Project, the
Paying Party [*] to the other Party in accordance with Section 10.7.3 or 10.7.4
hereof, as applicable, and shall be subject to the terms of Section 10.7.5(c)(i)
hereof with respect to [*] Partner Payments, except that the amount, if any,
paid by the Paying Party to the Non-Paying Party pursuant to this Section
10.7.5(c)(ii) [*] the amount due from the Paying Party to the Non-Paying Party
pursuant to Section 10.7.5(c)(i) hereof, if any. The obligation set forth in
this Section 10.7.5(c)(ii) shall [*] of any Commercial License that has been
granted in connection with the Partner Project to which such Collaboration
Product relates.
10.7.6 [*] on Account of Certain Antibody [*].
(a) It is understood and agreed that, in the event that a
Medarex Partner, during the course of development and/or commercialization of a
particular Collaboration Product against a particular Commercial Target,
substitutes for the Collaboration Antibody contained in such Collaboration
Product an Antibody raised against and with affinity for such Commercial Target
using Medarex Mice, from and after the date on which Medarex provides written
notice thereof to Kirin, [*] amounts received by Medarex from such Medarex
Partner in connection with such Antibody Product. In addition, in the event that
Medarex, prior to the Medarex Partner's making such substitution, received from
such Medarex Partner one or more Medarex Partner Payments related to the
achievement by such Medarex Partner of a milestone event(s) with respect to the
Collaboration Product (and Medarex made a corresponding payment(s) to Kirin
pursuant to Section 10.7.4 hereof), upon the Medarex Partner's achieving an
equivalent milestone event with respect to such modified Antibody Product,
Medarex shall provide notice thereof to Kirin [*] that corresponded to such
milestone event, [*] pursuant to Section 10.1.2 hereof with respect to such
Medarex Partner Payment. For the avoidance of doubt, in the event that the
Medarex Partner fails or otherwise does not achieve with respect to such
modified Antibody Product a milestone event equivalent to a milestone event
achieved with respect to the Collaboration Product prior to the Antibody
substitution (and with respect to which Medarex made a payment to Kirin pursuant
to Section 10.7.4 hereof), [*].
(b) It is further understood and agreed that, in the
event that a Third Party with which Medarex enters into a written agreement with
respect to a HuMAb Project, during the course of development and/or
commercialization of a particular Antibody Product containing an Antibody raised
against and with affinity for a particular Antigen using Medarex Mice, [*] for
such Antibody a Collaboration Antibody raised against and with affinity for such
Antigen using Kirin Mice and/or KM-Mice, thereby resulting in Medarex's
exercising rights, and/or granting a sublicense, under a Commercial License in
accordance with the terms and conditions of this Agreement, [*], Medarex shall
provide written notice thereof to Kirin and [*]. If, pursuant to the relevant
Project Agreement, [*] in connection with the Collaboration Product is [*] prior
to the [*], the amount [*].
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-110-
10.8 Equity Investments by a Party in Connection with Partner Projects.
10.8.1 In General. If a Contracting Party enters or is considering
entering into a Project Agreement governing a Partner Project pursuant to which
the Contracting Party would receive securities (other than in exchange for a
monetary payment at or above fair market value) from an Issuer as full or
partial consideration for the rights granted by the Contracting Party to the
Issuer pursuant to such agreement, and the issuance of such securities to the
Contracting Party in consideration for the respective rights would trigger a
corresponding payment obligation requiring the Contracting Party to make a
payment to the Non-Contracting Party pursuant to this Agreement based on the
consideration received for the respective rights, then the Contracting Party
shall have the right, at its election and subject to applicable law, to offer
the Non-Contracting Party the right to receive a portion of such securities in
lieu of cash in full or partial discharge (as the case may be) of the payment
obligation of the Contracting Party to the Non-Contracting Party pursuant to the
terms and conditions of this Article X. For the avoidance of doubt, the
Non-Contracting party shall in any event have the sole discretion to choose to
accept the Contracting Party's offer of a portion of such securities or to
reject the offer and receive only cash in payment of any and all obligations
hereunder.
(a) If, prior to entering into a Project Agreement that
provides for the issuance of securities, the Contracting Party desires to
determine whether the Non-Contracting Party would agree to receive a portion of
such securities in lieu of cash that would be due to the Non-Contracting Party
from the Contracting Party under this Agreement, the Contracting Party shall
provide written notice to that effect to the Non-Contracting Party, indicating
in such notice the identity of the Issuer, the unit price of the respective
securities and other relevant rights and preferences relating to such
securities. Further, if the Contracting Party has [*] issued or to be issued by
the Issuer, it shall [*] to the Non-Contracting Party; provided, however that
the Contracting Party shall [*] for the Non-Contracting Party unless any [*] of
the respective securities, in which case the Contracting Party shall promptly
provide the Non-Contracting Party with an amended notice reflecting the [*]. If
the Non-Contracting Party desires to receive a portion of such securities
(calculated according to Section 10.8.2 hereof) in lieu of cash in full or
partial discharge (as the case may be) of the payment obligation of the
Contracting Party to the Non-Contracting Party in connection with each such
issuance of securities to the Contracting Party, the Non-Contracting Party shall
provide written notice to the Contacting Party within ten (10) business days
from receipt by the Non-Contracting Party of the notice provided to it pursuant
to the first sentence of this Section 10.8.1(a) or in the event of an amended
notice provided as set forth in this Section 10.8.1(a), within [*] business days
from receipt by the Non-Contracting Party of such amended notice.
(b) If at any time after entering into a Project Agreement
that provides for the issuance of securities to the Contracting Party as
consideration for some or all of the rights granted by the Contracting Party to
the Issuer, the Contracting Party desires to offer a portion of such securities
to the Non-Contracting Party, the Contracting Party shall provide written notice
to the Non-Contracting Party, and inform the Non-Contracting Party of the
identity of the Issuer, the per unit price and other relevant rights and
preferences relating to such securities. If the Contracting Party has [*] issued
or to be issued by the Issuer, it shall [*] to the Non-Contracting Party;
provided, however that the Contracting Party shall [*] for the Non-Contracting
Party unless any [*] of the respective securities, in which case the Contracting
Party shall promptly provide the Non-Contracting Party with an amended notice
reflecting the [*]. In the event that the Non-Contracting Party desires to
obtain a separate valuation, it may do so [*]. If the Non-Contracting Party
desires to receive a portion of such securities (calculated according to Section
10.8.2 hereof) in lieu of cash in full or partial discharge (as the case may be)
of the payment obligation of the Contracting Party to the Non-Contracting Party
in connection with each such issuance of securities to the Contracting Party,
the Non-Contracting Party shall provide written notice to the Contacting Party
within [*] business [*] days from receipt of the notice provided by the
Contracting Party to the Non-Contracting
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-111-
Party pursuant to this Section 10.8.1(b) or in the event of an amended notice
provided as set forth in this Section 10.8.1(b), within [*] business [*]
business days from receipt by the Non-Contracting Party of such amended notice.
(c) In the event that the Non-Contracting Party provides
timely notice to the Contracting Party of its desire to receive a portion of
such securities pursuant to Section 10.8.1(a) or 10.8.1(b) hereof, then the
Contracting Party, at its election, shall attempt to negotiate an agreement with
the Issuer whereby the Issuer will issue directly to the Non-Contracting Party
securities in an amount calculated in accordance with Section 10.8.2 hereof, or
upon receipt by the Contracting Party of securities from the Issuer, the
Contracting Party shall take all steps reasonably within the control of the
Contracting Party to transfer to the Non-Contracting Party securities in an
amount calculated in accordance with Section 10.8.2 hereof. If (i) the Issuer
declines to issue such securities directly to the Non-Contracting Party or the
transfer of such securities by Contracting Party to Non-Contracting Party is not
permitted by the terms of its agreement with the Issuer and the Contracting
Party has requested that the Issuer consent to or waive any contractual
restrictions on the transfer of such securities by Contracting Party to
Non-Contracting Party, and the Issuer has declined to provide such consent
and/or waive such restrictions, as applicable, or (ii) the Contracting Party is
not permitted under applicable law to transfer such securities to the
Non-Contracting Party, then the Contracting Party shall have no further
obligation pursuant to this Section 10.8.1 and any payments owed by the
Contracting Party to the Non-Contracting Party in connection with the issuance
to the Contracting Party of such securities by the Issuer shall be paid by the
Contracting Party to the Non-Contracting Party in cash pursuant to the terms of
Section 10.13.1 hereof.
10.8.2 Calculation of Non-Contracting Party's Equity Interest. In the
event that the Non-Contracting Party elects pursuant to Section 10.8.1(a) or
10.8.1(b) hereof, as applicable, to receive a portion of the securities issued
by the Issuer to the Contracting Party as full or partial discharge (as the case
may be) of the payment obligation of the Contracting Party to the
Non-Contracting Party in connection with the rights granted in the Project
Agreement, and if the requirements of Section 10.8.1(c) are satisfied, then the
Non-Contracting Party shall receive a portion of any such securities issued to
the Contracting Party for which the Contracting Party has a corresponding
payment obligation to the Non-Contracting Party, which portion shall be the [*]
of [*] that equals the product of the [*] the [*] and a fraction, the numerator
of which shall be an amount equal to the [*] that [*] to the [*] pursuant to the
[*] of [*] on account of [*] to the [*] in connection with each payment, and the
denominator shall be the total cash value of the consideration (including
securities, cash or other value) provided by the Issuer to the Contracting Party
in connection with such payment. For purposes of this Section 10.8.2, the cash
value of securities and other non-cash assets shall equal the [*] in the [*]
that [*] to the Non-Contracting Party pursuant to this Section 10.8. Upon
request, the Contracting Party shall promptly provide the Non-Contracting Party
with [*] in such Contracting Party transaction.
10.8.3 No Obligation to Accept Equity. Nothing in this Section 10.8
shall require the Contracting Party to enter into an agreement with an Issuer,
or to accept securities from an Issuer as consideration for rights granted by
the Contracting Party to an Issuer pursuant to a Project Agreement.
10.9 In-House Project Royalty, Partner Royalty and Partner Payment Terms.
10.9.1 In-House Project Royalties. With respect to the royalty rates
for Collaboration Products resulting from In-House Projects, the Parties
acknowledge and agree that the Patent Rights and Know-How licensed pursuant to
this Agreement justify royalty rates of differing amounts with respect to sales
of such Collaboration Products, which rates could be applied separately to
Collaboration Products involving the exercise of such Patent Rights and/or the
incorporation of such Know-How, and that if such royalties were calculated
separately, royalties relating to Patent Rights and royalties relating to Know
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-112-
How would last for different terms. The Parties have determined in light of such
considerations and for reasons of convenience that blended royalty rates for the
Patent Rights and the Know-How licensed hereunder will apply during a single
royalty term (which blended royalty rates would be advantageous to both
Parties). Consequently, the Parties have agreed to adopt the royalty rates set
forth in Section 10.6 hereof. Each Party's respective royalty obligations under
Section 10.6 hereof shall terminate, on a country-by-country basis, with respect
to each Collaboration Product on the later to occur of (a) the expiration date
in such country of the last to expire of any issued patent that includes at
least one Valid Claim Controlled by the Party to which payment is owed that
covers the sale of such Collaboration Product or the use of the Collaboration
Mouse to obtain such Collaboration Product in such country, and (b) the [*] of
such Collaboration Product in such country, unless earlier terminated in
accordance with Article XIV hereof; provided, however, that in the event that
any such Collaboration Product is being [*] in the [*], if [*] to [*] amounts,
the [*] from its [*] after any applicable [*] and the [*] of the [*] of such [*]
has occurred in any such [*], once the applicable [*] such Party shall [*] have
an [*] to the [*] after the [*] of the [*] of [*] with respect to Net Sales of
such Collaboration Product in such country(ies).
10.9.2 Partner Royalties and Payments. Except as otherwise provided
in Section 10.1.2 hereof, each Party's respective obligations, under Sections
10.7.1 and 10.7.2 hereof with respect to Partner Royalties (and/or the payment
of certain [*] amounts related to sales of Collaboration Products by a Partner,
as the case may be) and under Sections 10.7.3 and 10.7.4 hereof with respect to
Partner Payments, shall apply with respect to [*] (and [*]) conducted pursuant
to [*] entered into by a Party (and/or its Affiliate) and a [*], and such [*]
shall [*] apply even in the event that this [*] (provided that such [*] a [*]
hereunder pursuant to Section [*] hereof (or a [*] a [*] pursuant to the terms
of the relevant [*], as applicable)); provided, however, that with respect to
any such Partner Project, each Party's respective obligations under Section
10.7.1, 10.7.2, 10.7.3 and 10.7.4 shall [*] apply [*] only for so long as such
[*] and [*] pursuant to the terms of such [*]. Notwithstanding anything
contained in this Agreement to the contrary, Kirin's obligation under Section
10.7.1(e) hereof, to [*] with respect to sales of each Collaboration Product
resulting from a Kirin Partner Project such [*] as the [*] with respect to such
sales, shall continue for so long as [*].
10.10 Partner Payments and Royalty Payments Periods. Amounts owed by a
Party to the other Party as Royalties on Net Sales of [*] Target Products and
[*] Target Products and on account of Partner Payments and Partner Royalties
shall be calculated in accordance with generally accepted accounting principles,
consistently applied, and with the terms of this Article X.
10.10.1 In-House Project Royalties. Royalties payable by one Party to
the other Party on Net Sales of [*] Target Products and [*] Target Products
shall be payable on a quarterly basis, within [*] days after the end of each
Calendar Quarter, based upon the Net Sales during such Calendar Quarter,
commencing with [*] of a Collaboration Product is made.
10.10.2 Partner Royalties and Payments. Amounts owed by a Party to
the other Party on account of Partner Payments and Partner Royalties shall be
payable on a [*], within [*] days after the [*] in which the paying Party
receives such Partner Payments and Partner Royalties from its Partner(s),
commencing with the [*] in which this Agreement becomes effective, except that
amounts owed by a Paying Party to the Non-Paying Party pursuant to Section
10.7.5 in connection with [*] Partner Payment obligations shall be payable as
provided in Sections 10.1.3, 10.7.5(c)(i) and 10.7.5(c)(ii) hereof, as
applicable.
10.11 Statements. Each payment made by a Party pursuant to this Agreement
shall be made in accordance with Section 10.13.1 hereof and shall be accompanied
by a statement that conforms to this Section 10.11. In the case of any payment
made by a wire service, the paying Party shall [*] by the
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-113-
paying Party. Each statement shall show in detail: (a) in the case of payments
on account of Partner Payments and Partner Royalties, (i) the amount of the
payment owed by the paying Party to the other Party, (ii) an identification of
the agreement pursuant to which the Partner Payments and Partner Royalties were
made, (iii) the Collaboration Product, if any, to which such payments relates,
and (iv) in the case of Partner Royalties, the calculation of royalties due from
the Partner to the Party, including the net sales calculation pursuant to the
Project Agreement governing such Partner Project; and (b) in the case of
royalties on Net Sales of any [*] Target Product or [*] Target Product, any (i)
Net Sales, (ii) the number of units of each Collaboration Product sold by the
paying Party (and its Affiliates, licensees, and sublicensees) on a
country-by-country basis during the applicable Calendar Quarter, and (iii) the
amount and calculation of royalties due on such Net Sales, including a
description of any and all offsets or credits deducted therefrom. In addition to
the information required by the preceding sentence, in the event that a Party
(or an Affiliate thereof) [*], which [*] constitutes a [*], in the case of any
such [*] received by Medarex (and/or its Affiliates), or a [*], in the case of
any such [*] received by Kirin (and/or its Affiliates), the Party receiving such
[*] from its [*] (either directly or indirectly through an Affiliate) shall
indicate in the corresponding statement provided to the other Party pursuant to
this Section 10.11 (x) the [*], and (y) the [*]. In the event of a disagreement
between the Parties with respect to the [*], the Party receiving such [*] from
its Partner (either directly or indirectly through its Affiliates) shall retain
a Third Party, acceptable to the other Party, to perform [*], the [*] by the
Parties, and the results of which shall be binding on the Parties.
Notwithstanding anything contained in this Section 10.11, in the event that a
Party receives any [*], if the Project Agreement provides for the [*] in such
[*] shall be [*] for purposes of this Agreement.
10.12 Records Retention; Audit.
10.12.1 Record Retention. Until the third (3rd) anniversary of the
Calendar Quarter in which a Collaboration Product is sold, the selling Party
shall keep (and shall ensure that its Affiliates and, with respect to any
Project Agreements governing Partner Projects and/or In-House Projects and is
entered into after the Effective Date, its Partners and In-House Collaborators,
shall keep) records of such sales in sufficient detail to confirm the accuracy
of the payments made by a Party to the other Party pursuant to this Article X.
10.12.2 Audit. Upon the written request of the receiving Party and
[*], the paying Party shall permit an independent certified public accounting
firm of nationally recognized standing selected by the receiving Party, and
reasonably acceptable to the paying Party, at the receiving Party's expense, to
have access during normal business hours, and upon reasonable prior written
notice, to such of the records of the paying Party as may be reasonably
necessary to verify the accuracy of the payments made by the paying Party to the
other Party hereunder for any Calendar Year ending [*] to the date of such
request. The accounting firm shall disclose to the paying Party and the
receiving Party whether the payments are correct or incorrect and the specific
details concerning any discrepancies, including any underlying data to the
extent that the receiving Party may reasonably require access to it in order to
enforce its rights under this Agreement.
10.12.3 Payment of Additional Amounts. If such accounting firm
concludes that additional amounts were owed during such period, the paying Party
shall pay the additional amounts, with interest from the date originally due at
the rate of one and a half percent (1.5%) per month, within [*] days after the
date on which such accounting firm's written report is delivered to the paying
Party; provided however, if the payment of interest at such rate is not
permitted or enforceable under the laws or regulations of [*], the paying Party
shall be deemed to satisfy the interest requirement under this Section by paying
to the other Party the maximum interest attainable by law for the applicable
period. If, and
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-114-
only if, the amount of the underpayment is five percent (5%) or greater for the
period under audit, then the paying Party shall reimburse the receiving Party
for all costs related to such audit.
10.12.4 Confidentiality. Except to the extent reasonably necessary
to enforce its rights under this Agreement, the receiving Party shall treat all
information subject to review under this Section 10.12 in accordance with the
confidentiality provisions of Article XIII and shall cause the accounting firm
conducting the audit under this Section 10.12 to enter into a reasonably
acceptable confidentiality agreement with the paying Party obligating such firm
to retain all such financial information in confidence pursuant to such
confidentiality agreement.
10.13 Mode of Payment and Withholding and Similar Taxes.
10.13.1 Mode of Payment. Except for payments made in the form of
securities pursuant to Section 10.8 hereof, all payments by either of the
Parties to the other Party under this Agreement shall be made [*] in the
requisite amount to such [*] as the receiving Party may from time to time
designate by notice to the paying Party, calculated as necessary pursuant to
Section 10.14 hereof. For purposes of Section 10.8 hereof, the Contracting Party
shall promptly (or shall cause Issuer to promptly) transfer to Non-Contracting
Party the appropriate certificates and/or other forms of documentation
evidencing the portion of the securities issued by Issuer to Contracting Party
which the Non-Contracting Party has elected to receive thereunder.
10.13.2 Withholding and Similar Taxes. Notwithstanding any provision
of this Agreement, all payments remitted by either of the Parties to the other
Party will be reduced by the applicable withholding taxes or similar charges
imposed by any government in the applicable taxing jurisdiction and proof of
payment of such taxes or charges shall be secured and sent to the such other
Party as evidence of such payment. All amounts paid by a Party pursuant to this
Section 10.13.2 shall be paid for the account of such other Party and deducted
from the amounts due to such other Party pursuant to the terms of this
Agreement.
10.14 Payments in Other Currencies. Subject to Sections 10.1.3, 10.2.2,
10.3.1(b), and 10.7.5(c), in connection with royalties on sales of [*] Target
Products and [*] Target Products outside the United States, and payments between
the Parties on account of Partner Payments and Partner Royalties received by a
Party from a Partner in currencies other than United States Dollars, payments
between the Parties shall be calculated based on the [*] (a) in which such sales
occur, in the case of royalties on sales of [*] Target Products and [*] Target
Products, and (b) in which such Partner Payments and Partner Royalties are
received by the Party from a Partner, in the case of Partner Payments and
Partner Royalties. If by law, regulation, or fiscal policy of a particular
country, conversion into United States dollars or transfer of funds of a
convertible currency to the United States is restricted or forbidden, the paying
Party shall give the receiving Party prompt written notice of such restriction,
which notice shall satisfy the [*] payment deadline described in Section
10.10.1, 10.10.2 or 10.12.3 hereof, as applicable. The paying Party shall pay
any amounts due to the receiving Party by depositing such payment in local
currency to the credit of the receiving Party in a recognized banking
institution or trust company selected by the receiving Party and identified by
written notice to the paying Party, and such deposit shall fulfill all
obligations of the paying Party to the receiving Party with respect to such
payment.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-115-
ARTICLE XI
RESEARCH AND COLLABORATION RELATING TO JOINT TECHNOLOGY
11.1 Research and Research Reports. Medarex and Kirin acknowledge and
agree that, in accordance with Section 16(a) of the Agreement on Essential Terms
for Collaboration, Medarex has conducted research relating to KM-Mice and has
provided to Kirin four reports relating to Medarex's research findings, with the
final report having been delivered by Medarex to Kirin on or about September 30,
2001. As a result of Medarex's submission to Kirin of such reports, the entire
Initial Fee has been fully earned by Medarex and no portion of such fee shall be
refundable or creditable.
11.2 Collaboration Meetings for Development of Medarex, Kirin and
Joint Technologies. During the Term of this Agreement, the Parties shall
participate in biannual joint meetings to confer on the collaboration under this
Agreement with respect to the Kirin Technology, Medarex Technology, and
technology relating to KM-Mice, KM Patent Rights, and KM Know-How, and regarding
the continued efforts of the Parties, if any, with respect to the development of
the Technologies licensed pursuant to this Agreement. To the extent required
pursuant to Section 6.5.2 hereof, and to the extent permitted by applicable law
and the terms of applicable agreements with Third Parties, each Party's
representative shall share with the other Party's representative: (a) general
information regarding any Project Agreement governing one or more Partner
Projects and/or In-House Projects, (and, in the case of Medarex, HuMAb License
Projects and HuMAb Collaboration Agreements) entered into with a Third Party;
(b) the identity of such Third Party; (c) general information about systems
being utilized by such Third Party to develop products, whether or not such
development is complete; and (d) the general nature of any products developed by
such Third Party through use of technology licensed to it under such agreement.
Any information shared by a Party's representative with the other Party's
representative pursuant to this Section 11.2 shall be deemed Confidential
Information of the disclosing Party subject to the terms and conditions of
Article XIII hereof. The designated location for such meeting shall alternate
between Japan and the United States, or as otherwise agreed by the Parties, and
shall be attended by one or more representatives of both Kirin and Medarex as
each Party shall in its sole discretion determine. Each Party shall bear its own
expenses relating to attendance at and participation in joint meetings.
ARTICLE XII
INTELLECTUAL PROPERTY RIGHTS
12.1 Intellectual Property Ownership.
12.1.1 Ownership of Medarex Technology and Medarex Trademarks.
This Agreement shall not affect the extent of Medarex's ownership of the Medarex
Mice (and any Mice Materials derived therefrom), Medarex Patent Rights, Medarex
Know-How, and Medarex Trademarks. Kirin hereby admits the validity of the
Medarex Trademarks, agrees not to challenge the Medarex Trademarks, and agrees
that any and all rights that may be acquired by the use of the Medarex
Trademarks, including any goodwill symbolized thereby, shall inure to the sole
benefit of Medarex.
12.1.2 Ownership of Kirin Technology and Kirin Trademarks. This
Agreement shall not affect the extent of Kirin's ownership of the Kirin Mice
(and any Mice Materials derived therefrom), Kirin Patent Rights, Kirin Know-How,
and Kirin Trademarks. Medarex hereby admits the validity of the Kirin
Trademarks, agrees not to challenge the Kirin Trademarks, and agrees that any
and all rights that may be acquired by the use of the Kirin Trademarks,
including any goodwill symbolized thereby, shall inure to the sole benefit of
Kirin.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-116-
12.1.3 Ownership of Improvements. Except as set forth in Section
12.1.4 hereof, and without the payment of additional consideration, (a) Medarex
agrees to disclose promptly in writing and assign to Kirin any and all
intellectual property rights of Medarex in or to Improvements to the TC Mice or
HAC Mice (but excluding any such Improvement that also constitutes an
Improvement to the Medarex Mice) that are (i) conceived, reduced to practice, or
otherwise developed by or on behalf of Medarex or any Medarex Affiliate during
the period beginning on December 27, 1999, and ending on the last day of the
Term, or (ii) assigned to Medarex by any of its Partners or In-House
Collaborators in accordance with the terms and conditions of Section 8.6.3(a)
hereof; and (b) Kirin agrees to disclose promptly in writing and assign to
Medarex, without the payment of additional consideration, any and all
intellectual property rights of Kirin in or to Improvements to the Medarex Mice
(but excluding any such Improvement that also constitutes an Improvement to the
TC Mice or HAC Mice) that are (i) conceived, reduced to practice, or otherwise
developed by or on behalf of Kirin or any Kirin Affiliate during the period
beginning on December 27, 1999, and ending on the last day of the Term, or (ii)
assigned to Kirin by any of its Partners or In-House Collaborators in accordance
with the terms and conditions of Section 8.6.3(a) hereof. It is understood and
agreed, however, that in no event shall any (x) Collaboration Antibody,
Collaboration Product, or Production Process Development, (y) Information and
Inventions that would fall within the definition of "Excluded Claims" or
"Excluded Know-How", or (z) KM-MouseTM or any Improvement thereto, conceived,
reduced to practice, or otherwise developed by or on behalf of a Party (or any
of its sublicensees) be deemed to be an Improvement to the TC Mice, HAC Mice or
Medarex Mice subject to the assignment obligation set forth in this Section
12.1.3.
12.1.4 Ownership of KM Patent Rights, KM Know-How and KM
Trademarks. As between the Parties, the Parties shall jointly and equally own
any and all right, title, and interest in or to any KM Trademarks, KM Patent
Rights and KM Know-How, and any Improvements to any of the KM Patent Rights and
KM Know-How conceived, reduced to practice, or otherwise developed by or on
behalf of either Party, or that are assigned to either Party by any of such
Party's Partners or In-House Collaborators (or employees thereof) in accordance
with the terms and conditions of Section 8.6.3(a) hereof; provided, however,
that except as expressly permitted by the terms of this Agreement, neither Party
may Exploit or disclose to or share with any Third Party any KM-MouseTM, KM
Patent Rights, or KM Know-How during the Term of this Agreement without the
express written consent of the other Party. Each Party agrees to disclose
promptly in writing to the other Party any and all KM Know-How and KM Patent
Rights, that are (a) conceived, reduced to practice, or otherwise developed by
or on behalf of such Party during the period beginning on December 27, 1999, and
ending on the last day of the Term, or (b) assigned to such Party by any of its
Partners or In-House Collaborators (or employees thereof) in accordance with the
terms and conditions of Section 8.6.3(a) hereof. In addition, the Party with an
obligation to make such disclosures agrees, as necessary to evidence joint
ownership of any and all such KM Patent Rights, to assign to the other Party,
and/or to cause its employees to assign to the other Party, without payment of
additional consideration, an equal, undivided interest in such KM Patent Rights
(and the Information and Inventions on which any such KM Patent Rights are
based). It is understood and agreed, however, that in no event shall any (y)
Collaboration Antibody, Collaboration Product, or Production Process
Development, or (z) Information and Inventions that would fall within the
definition of "Excluded Claims" or "Excluded Know-How", be deemed to be subject
to the assignment obligation set forth in this Section 12.1.4.
12.1.5 Ownership of Antibodies and Antibody Products. Subject to
Section 12.2.5 hereof, as between the Parties, each Party shall own all right,
title, and interest in and to any Antibodies, Antibody Products, Antibody
Materials, and Information and Inventions related to any of the foregoing,
invented, discovered, or developed by such Party resulting from the use of
Collaboration Mice pursuant to this Agreement.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-117-
12.1.6 Ownership of Production Process Technology.
Notwithstanding the foregoing, each Party shall own and retain all right, title
and interest in and to such Party's Production Process Technology, including any
and all Information and Inventions with respect to such Production Process
Technology (including any Improvements thereto) that are conceived, reduced to
practice, discovered, developed or otherwise made, by or on behalf of such
Party, its Affiliates or its sublicensees, whether or not patented or
patentable, and any and all Patent Rights and other intellectual property rights
with respect thereto. Except as the Parties may otherwise expressly agree,
neither Party shall have any rights, express or implied, under this Agreement
with respect to any Production Process Technology of the other Party and nothing
in this Agreement is intended to or shall be interpreted as granting a Party any
license to such Production Process Technology, whether subordinate or dominant
to any other Technology.
12.2 Prosecution of Patents and Trademarks.
12.2.1 Medarex Rights.
(a) In General. As between the Parties, Medarex shall
have the sole right, at its sole cost and expense, to prepare, file, prosecute,
and maintain throughout the world (and otherwise control the conduct of any
proceedings before any patent official or patent office with respect to) (i) the
Medarex Patent Rights; (ii) [*] the Patent Rights relating to Antibodies,
Antibody Materials, and Antibody Products invented, discovered, or developed by
Medarex or any of its sublicensees resulting from the use of Collaboration Mice
pursuant to this Agreement; (iii) the Patent Rights relating to Medarex's
Production Process Technology; and (iv) registration of the Medarex Trademarks.
In addition, Medarex shall have the sole right, but not the obligation, at its
expense, to prepare, file, prosecute, and maintain (and otherwise control the
conduct of any proceedings before any patent official or patent office with
respect to) Patent Rights relating to any Improvements assigned by Kirin to
Medarex pursuant to Section 12.1.3 hereof.
(b) Certain Medarex Patent Rights. Notwithstanding the
foregoing terms of Section 12.2.1(a), in the event that Medarex files a patent
application that covers any Medarex Patent Rights and determines subsequent to
making the filing that Medarex no longer desires to prosecute and/or maintain
any such Medarex Patent Rights, Medarex shall promptly (which, in any event,
shall be [*]) notify Kirin in writing of such decision and identify the Medarex
Patent Rights at issue [*] If [*], Kirin shall provide written notice thereof to
Medarex within [*] days after the receipt by Kirin of notice from Medarex
pursuant to this Section 12.2.1(b). Upon receipt of timely notice from Kirin,
Medarex shall [*]. Absent timely notice from Kirin, Medarex shall [*].
12.2.2 Kirin Rights.
(a) In General. As between the Parties, Kirin shall have
the sole right, at its sole cost and expense, to prepare, file, prosecute, and
maintain throughout the world (and otherwise control the conduct of any
proceedings before any patent official or patent office with respect to) (i) the
Kirin Patent Rights; (ii) subject to Section 12.2.5 hereof, the Patent Rights
relating to Antibodies, Antibody Materials, and Antibody Products invented,
discovered, or developed by Kirin or any of its sublicensees resulting from the
use of Collaboration Mice pursuant to this Agreement; (iii) the Patent Rights
relating to Kirin's Production Process Technology; and (iv) registration of the
Kirin Trademarks. In addition, Kirin shall have the sole right, but not the
obligation, at its expense, to prepare, file, prosecute, and maintain (and
otherwise control the conduct of any proceedings before any patent official or
patent office with respect to) Patent Rights relating to any Improvements
assigned by Medarex to Kirin pursuant to Section 12.1.3 hereof.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-118-
(b) Certain Kirin Patent Rights. Notwithstanding the
foregoing terms of Section 12.2.2(a), in the event that Kirin files a patent
application that covers any Kirin Patent Rights and determines subsequent to
making the filing that Kirin no longer desires to prosecute and/or maintain any
such Kirin Patent Rights, Kirin shall promptly (which, in any event, shall be
[*] notify Medarex in writing of such decision and identify the Kirin Patent
Rights at issue [*]. If [*], Medarex shall provide written notice thereof to
Kirin within [*] days after the receipt by Medarex of notice from Kirin pursuant
to this Section 12.2.2(b). Upon receipt of timely notice from Medarex, Kirin
shall [*]. Absent timely notice from Medarex, Kirin shall [*].
12.2.3 [*] Certain Medarex Patent Rights and Certain Kirin
Patent Rights [*]. In the event that Medarex is [*] pursuant to Section
12.2.1(b) to [*] certain Medarex Patent Rights, or that Kirin is [*] pursuant to
Section 12.2.2(b) to [*] certain Kirin Patent Rights, the Responsible Party
shall [*] with respect to the [*] such Patent Rights. Such [*] includes, without
limitation, (a) [*] the Non-Responsible Party [*] provision of a copy of any
official correspondence received by such Party from a [*] with respect to such
Patent Rights; and (b) providing the Non-Responsible Party a [*] on any [*] to a
[*] at least [*] days prior to [*], unless such [*] allows [*] for the
Responsible Party to [*], in which case the Party shall provide the other Party
an [*] on any [*] to such [*] days prior to [*]. If the Non-Responsible Party
fails to [*] days after receiving such [*], or, in the event [*], to provide
comments within [*] days after receiving such [*], the [*]. If, however, the
Non-Responsible Party [*] with respect to such [*], the Responsible Party agrees
to [*], it being understood, however, that the Responsible Party shall have the
right to [*]. At any time during the [*] any Patent Rights pursuant to this
Section 12.2.3, the Non-Responsible Party may inform the Responsible Party that
it [*] associated with such activities, in which case the Non-Responsible Party
shall [*] the Responsible Party [*] after receipt of such notice and the
Responsible Party shall have [*].
12.2.4 KM Patent Rights and KM Trademarks.
(a) In General. Subject to Section 12.2.5 hereof, the
Parties shall have the following rights and obligations with respect to the
preparation, filing, prosecution and maintenance of any and all KM Patent Rights
and registrations of any and all KM Trademarks throughout the world. With
respect to the preparation, filing, prosecution, and maintenance of such KM
Patent Rights or registrations of KM Trademarks (and any proceedings relating
thereto before any trademark or patent official or office) in any country or
jurisdiction, the Party having the first right, but not the obligation, to
conduct and control such activities shall be the Party in whose Primary
Promotional Area the country or jurisdiction is located. If a Party elects not
to prepare, file, prosecute and/or maintain any KM Patent Rights or
registrations of KM Trademarks (or otherwise control the conduct of proceedings
with respect thereto before any trademark or patent official or office) in a
particular country within the Party's Primary Promotional Area, then such Party
shall give the other Party written notice of such election [*] days before any
right would be forfeited if no action was taken, and the other Party shall
thereafter have the right, but not the obligation to prepare, file, prosecute
and/or maintain such KM Patent Rights or registrations of KM Trademarks (or
otherwise control the conduct of proceedings with respect thereto before any
trademark or patent official or office). Medarex and Kirin shall assist and
cooperate with one another in, and share equally the cost and expense of,
preparing, filing, prosecuting, and maintaining the KM Patent Rights and
registrations of KM Trademarks. Notwithstanding the foregoing, either Party may
decline to pay its share of costs for preparing, filing, prosecuting, and
maintaining any KM Patent Rights or registrations of KM Trademarks (or
conducting other proceedings relating to such Patent Rights or Trademarks before
any trademark or patent official or office) in a particular country or
particular countries, in which case the declining Party shall assign, and shall
cause its Affiliates to assign, if necessary, to the other Party all of
its/their right, title, and interest in and to any such KM Patent Rights and KM
Trademarks in the relevant country or countries, whereupon such KM Patent Rights
and KM
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-119-
Trademarks shall become part of Medarex's Patent Rights and Medarex Trademarks
(in the case of an assignment by Kirin) or Kirin's Patent Rights and Kirin
Trademarks (in the case of an assignment by Medarex) in such country or
countries, as the case may be.
(b) Cooperation. Each Party shall, subject to Section
12.2.5 hereof, cooperate fully in the other Party's preparation, filing,
prosecution, and maintenance of the KM Patent Rights and registrations of KM
Trademarks (and in any other proceedings before a trademark or patent official
or office with respect thereto). Such cooperation includes, without limitation,
(i) promptly executing all papers and instruments or requiring employees to
execute such papers and instruments as reasonable and appropriate so as to
enable such Party to prepare, file, prosecute, and maintain its KM Patent Rights
and registrations of KM Trademarks in any country; and (ii) [*] the other Party
[*] provision of a copy of any official correspondence received by such Party
from a trademark or patent office in any country with respect to KM Patent
Rights or KM Trademarks. In addition, with respect to any Patent Rights within
KM Patent Rights, for so long as such patent remains jointly owned, the Party
having prosecution responsibility therefor agrees to provide the other Party a
[*] to [*] and [*] any [*] to a [*] at least [*] days [*] to [*], unless such
[*] allows less than [*] days for the Party having prosecution responsibility to
[*], in which case the Party shall provide the other Party an [*] and [*] any
[*] to such [*] at least [*] days [*] to [*]. If the other Party fails to [*]
days after receiving such [*], or, in the event the [*] allows less than [*]
days for the Responsible Party to [*], to provide comments within [*] days after
receiving sucsh [*], the [*]. If, however, the other Party [*] with respect to
[*], the responsible Party agrees to [*], it being understood, however, that the
responsible Party shall have the right, subject to Section 12.2.5 hereof, to
[*].
12.2.5 Use of Certain Mice and Certain Data in Connection with
Patent Filings. Notwithstanding the rights conferred on each Party by Section
12.2.4 hereof with respect to the preparation, filing, prosecution, and
maintenance of KM Patent Rights, on Kirin, pursuant to Section 12.2.2 hereof,
with respect to Kirin's preparation, filing, prosecution, and maintenance of
Kirin Patent Rights and on Medarex, pursuant to Section 12.2.1 hereof, with
respect to Medarex's preparation, filing, prosecution, or maintenance of Medarex
Patent Rights, (i) Medarex shall not, in any filing, correspondence or other
submission to any patent official or patent office, (A) include [*], or (B)
describe the [*] (other than in connection with claims that fall exclusively
within the Excluded Claims), in each case without providing Kirin with an
opportunity to [*] of any such filing, correspondence or other submission and
[*], and (ii) Kirin shall not, in any filing, correspondence or other submission
to any patent official or patent office, (A) include [*], or (B) describe the
[*] (other than in connection with claims that fall exclusively within the
Excluded Claims), in each case without providing Medarex with an opportunity to
[*] of any such filing, correspondence or other submission and [*].
Notwithstanding the foregoing, in the event that data derived from the [*] for
Kirin's [*] preparation, filing, prosecution, or maintenance of Kirin Patent
Rights or KM Patent Rights, Medarex shall [*] to Kirin's use of such data, and
in the event that data derived from [*] for Medarex's [*] preparation, filing,
prosecution, or maintenance of Medarex Patent Rights or KM Patent Rights, Kirin
shall [*] to Medarex's use of such data.
12.2.6 Patent Filings. Subject to Section 12.2.4 hereof and the
last sentence of this Section 12.2.6, except as otherwise provided in this
Section, Kirin covenants not to, and to cause its Affiliates not to, file any
patent application (including, without limitation, a PCT application) describing
or claiming Information and Inventions containing any Medarex Mice or KM-Mice or
use thereof, without Medarex's prior written consent. Subject to Section 12.2.4
hereof and the last sentence in this Section 12.2.6, Medarex covenants not to,
and to cause its Affiliates not to, file any patent application (including,
without limitation, a PCT application) describing or claiming Information or
Inventions containing any Kirin Mice or KM-Mice or use thereof, without Kirin's
prior written consent. In addition,
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-120-
except as otherwise provided in this Section, each Party agrees that [*], it
will [*] include in its Project Agreements governing Partner Projects and/or
In-House Projects, terms that require the Partner or In-House Collaborator, as
the case may be, to refrain from filing any patent application describing or
claiming Information or Inventions containing, in the case of a Partner or
In-House Collaborator of Kirin, any Medarex Mice or KM-Mice, and in the case of
a Partner or In-House Collaborator of Medarex, any Kirin Mice or KM-Mice.
Notwithstanding the foregoing, [*] Party [*] for the filing by such Party or its
Affiliate (and, subject to Section 8.6.1(b), neither Party has [*] Partners or
In-House Collaborators to [*]) of a patent application on an Antibody, Antibody
Product or Antibody Material, Production Process Development, Manufacturing
technology or the use of any of the foregoing.
12.3 Enforcement of Patents.
12.3.1 Rights and Procedures. If either Party determines that any of
the Patent Rights or Licensed Trademarks subject to this Agreement may be the
subject of infringement by a Third Party, it shall [*] days after becoming aware
of such infringement by a Third Party, notify the other Party in writing and
[*].
(a) Medarex Technology, Medarex Trademarks, Kirin Technology
and Kirin Trademarks. Medarex shall have the sole right, but not the obligation,
[*] to attempt to ▇▇▇▇▇ such infringement, including by filing an infringement
suit or taking other similar action, to the extent such infringement relates to
Medarex Technology or Medarex Trademarks. Kirin shall have the sole right, but
not the obligation, [*] to attempt to ▇▇▇▇▇ such infringement, including by
filing an infringement suit or taking other similar action, to the extent such
infringement relates to the Kirin Technology or Kirin Trademarks. If required by
law in order for a Party to prosecute such an infringement suit pursuant to this
Section 12.3.1(a), the licensee Party shall join such a suit as a party, and
whether or not joined as a party shall provide such assistance [*] by the other
Party in relation thereto, in each case at the [*]. Notwithstanding anything
contained in this Section 12.3.1(a), it is understood and agreed that neither
Party shall have any obligation to file an infringement suit or take other legal
action against any Third Party based on claims of infringement of such Party's
Patent Rights or Trademarks.
(i) If the Party that Controls the Technology alleged
to be infringed declines or otherwise fails to bring a patent infringement suit
to ▇▇▇▇▇ any such alleged patent infringement in one or more countries where
such alleged infringement is occurring, then the licensee Party shall have the
right on a [*] basis to [*] any [*] that it is required to [*] pursuant to
Section [*] hereof, and any [*] that it is required to [*] pursuant to Section
[*] or [*] hereof, as applicable, in each case with respect to a Collaboration
Product commercialized by the licensee Party (or its sublicensee), which
Collaboration Product (A) was raised against and has a useful affinity for the
same Antigen for which the Third Party's product has a useful affinity, and (B)
is intended for an application (e.g., In Vivo Therapeutic Application, Ex Vivo
Therapeutic Application, Diagnostic Application or use as a Regent) that is the
same application for which the Third Party's product is intended, if in such
country, and for so long as, all of the following conditions are satisfied (as
demonstrated by [*]:
(a) The licensee Party provides to the licensor
Party a [*] (which [*]), concluding that there is a [*] for the [*] on the
ground that a [*]; and
(b) The Third Party's product shall [*]; and
(c) To the extent that the licensee Party (or its
sublicensee) [*], then the licensee Party (or its sublicensee) shall have [*],
unless the licensee Party (or its sublicensee) has already [*].
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-121-
(ii) In addition to the rights provided to a
licensee Party pursuant to Section 12.3.1(a)(i), with respect to any
Collaboration Product(s) for which the licensee Party has satisfied the
conditions provided in Sections 12.3.1(a)(i)(a), 12.3.1(a)(i)(b) and
12.3.1(a)(i)(c) hereof [*], for [*], if the Third Party's [*] product achieve
[*]), the licensor Party shall [*] the licensee Party for each such [*] for such
Collaboration Product(s), in the case of a Collaboration Product(s) resulting
from an In-House Project, or (z) [*] for such Collaboration Product(s). For the
avoidance of doubt, once any [*].
(iii) Notwithstanding anything contained in Sections
12.3.1(a)(i) or 12.3.1(a)(ii) hereof, neither Party shall [*] in connection with
any Collaboration Product commercialized by such Party's Partner pursuant to a
Project Agreement [*] to the [*] that such [*] to such Party or [*] of [*] such
Party pursuant to the terms of the [*], as the case may be.
(b) KM Patent Rights and KM Trademarks. In the case of any
infringement by a Third Party that relates to the KM Patent Rights or KM
Trademarks, the Responsible Party shall have the right, but not the obligation,
[*] (unless a [*] is otherwise [*] the Parties), to attempt to ▇▇▇▇▇ such
infringement by commercially appropriate steps, including filing an infringement
suit or taking other similar action. In the event that the Responsible Party
elects to not take any legal action with respect to such Third Party
infringement, within [*] days following notice by one Party to the other Party
of the claim of infringement, the Acting Party shall have the right but not the
obligation, [*] (unless a [*] is otherwise [*] the Parties), to act to ▇▇▇▇▇ any
such Third Party infringement, including, without limitation, by prosecuting an
infringement suit or other legal proceeding. Notwithstanding the foregoing, each
Party shall fully cooperate with the other Party in any action to ▇▇▇▇▇
infringement of the KM Patent Rights or KM Trademarks, and, if required by law
in order for a Party to prosecute such suit, shall join such suit as a party.
Notwithstanding anything to the contrary herein, each Party agrees that it shall
[*] with respect to any KM Patent Rights or KM Trademarks for which it has
responsibility [*], which [*].
12.3.2 Costs and Expenses. Any amounts recovered by either Party
pursuant to Section 12.3.1(a) hereof or 12.3.1(b) hereof, whether by settlement
or judgment, shall be applied in the following order of priority: (a) to [*]
each Party for its [*] and [*] (which amounts shall be allocated [*]); (b) to
[*] each Party for any amounts that such Party is [*] in respect of such [*]
pursuant to applicable [*] pursuant to which such [*] was [*] or other [*]
and/or [*]; and (c) the remainder to be [*] who [*], except that in the case of
any [*] by [*] or its [*], such amounts shall be allocated [*] based on the [*]
as reflected in the terms and conditions of [*].
12.4 Infringement of Third Party Rights.
12.4.1 Third Party Patent Licenses.
(a) In General. Each Party shall have the right (but not the
obligation) to negotiate and obtain from any Third Party any license or other
rights that such Party deems appropriate, including a license under any Core
Third Party Patent, in order to permit the full and unhindered use, subject to
the terms and conditions of this Agreement, of any Collaboration Mice (and any
Improvements thereto) and the practice of any of the Patent Rights licensed
hereunder. Except as expressly provided in Section 12.4.1(b) hereof and the
other terms and conditions of this Agreement, (i) each Party shall be [*] for
the [*] to any [*] for [*], including any [*] to [*] that [*] by the [*], and/or
the [*], and shall [*] to the other Party pursuant to this Agreement; and (ii)
if the Party that obtains a license from a Third Party grants a sublicense to
the other Party (and the other Party's sublicensees), such sublicense shall be
subject to the [*], including through the use of any Collaboration Mice and/or
the Exploitation of any Collaboration Antibodies or Collaboration Products.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-122-
(b) Core Third Party Patents. If either Party becomes aware of
any Core Third Party Patent during the term of this Agreement, or any pending
patent application that would be a Core Third Party Patent if issued, such Party
shall [*]. If a Party obtains from a Third Party a license under any Core Third
Party Patent(s), at the time that the Party obtains the license, it shall [*] to
[*] to the [*] (with the [*] to the [*]). If a Party obtains a [*] any [*] it
shall [*]. The other Party shall [*].
(i) If a Party obtains a license from a Third Party under
Core Third Party Patents in order to be able to use the other Party's mice, the
KM-Mice (to the extent that the Core Third Party Patent would be infringed, in
the absence of the license, as a result of any crossbreeding with the other
Party's mice or the incorporation of the other Party's Technology in such
KM-Mice) or to practice the other Party's Technology or the KM Patent Rights (to
the extent that the Core Third Party Patent would be infringed, in the absence
of the license, as a result of any crossbreeding with the other Party's mice or
the incorporation of the other Party's Technology in the KM-Mice) without
infringement, and in each case excluding any Third Party Patent Rights to the
extent such Patent Rights [*], the Party obtaining the license shall have the
right, on a [*] basis, to [*] of the [*] on [*] (whether in the form of [*], or
as a [*]) to be [*] to the other Party under this Agreement on sales of such
Collaboration Product in such country, subject to Section 12.4.1(b)(iii) hereof.
If a Party claims the right to [*] pursuant to this Section 12.4.1(b)(i), it
shall [*] to the [*], which [*] at issue and the [*] for its [*].
(ii) If a Party obtains a license from a Third Party under
Core Third Party Patents in order to be able to use its own mice, the KM-Mice
(to the extent that the Core Third Party Patent would be infringed, in the
absence of the license, as a result of crossbreeding with the Party's own mice
or the incorporation of the Party's own Technology in such KM-Mice) or to
practice its own Technology or the KM Patent Rights (to the extent that the Core
Third Party Patent would be infringed, in the absence of the license, as a
result of crossbreeding with the Party's own mice or the incorporation of the
Party's own Technology in the KM-Mice) without infringement, and in each case
excluding any Third Party Patent Rights to the extent they claim [*], such Party
shall [*] on account of such Party's [*] and such Party shall [*] to [*] against
the [*] under this Agreement. If the Party obtaining the license [*], the Party
that [*] shall be [*] to the [*] on account of [*] with respect to such [*];
provided, however, that such other Party shall have the right on a [*] basis, to
[*] in connection with the [*] (whether in the form of [*], or as a [*]) to be
paid by such other Party under this Agreement on sales of such Collaboration
Product in such [*], subject to Section 12.4.1(b)(iii) hereof.
(iii) Notwithstanding anything contained in Section
12.4.1(b)(i) or 12.4.1(b)(ii) hereof, [*] (whether as [*], or as a [*]) when [*]
under this Agreement, regardless of the amount of any [*], shall be [*] in any
[*]; provided, however, that [*] otherwise allowed under Section 12.4.1(b)(i) or
12.4.1(b)(ii) hereof [*] may be [*], subject to this Section 12.4.1(b)(iii).
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-123-
12.4.2 Third Party Litigation.
(a) Infringement Claims Relating to Medarex Technology,
Medarex Trademarks, Kirin Technology and/or Kirin Trademarks. Subject to
Sections 12.4.2(b) and 12.4.2(d) hereof, in the event that a Third Party
institutes a Third Party IP Infringement Suit relating to either Party's
Technology or Licensed Trademarks, then the Party that is named as the defendant
shall have [*] asserted against such Party, including the right to [*], except
as set forth herein. The Party defending such suit shall [*]. The Party
defending the Third Party IP Infringement Suit shall make [*] with respect to
any Patent Rights or Know-How owned by the other Party or jointly owned by both
Parties [*] with the other Party. If the Third Party IP Infringement Suit
involves [*], in addition to the actions required above, the Party defending
such claims shall (i) [*] to the other Party, (ii) provide [*] to the other
Party of [*], and obtain from such other Party [*] to the extent that it [*],
which [*], and (iii) [*], except in the context of a [*] pursuant to this
Section 12.4.2(a), [*] or otherwise [*] without [*] and an [*].
(b) Infringement Claims Relating to Jointly-Owned Intellectual
Property. The Parties shall have the right to jointly direct and control the
defense of a Third Party IP Infringement Suit to the extent the claims involve
jointly-owned intellectual property (i.e., KM Patent Rights, KM Know-How, or KM
Trademarks). In the event that a Third Party institutes a Third Party IP
Infringement Suit against a Party or any of its Affiliates that includes claims
that involve jointly-owned intellectual property, and [*] but shall continue to
provide to [*]. Regardless of whether such other Party [*], the Party defending
such claims shall (i) [*] to the other Party, (ii) provide [*] to the other
Party of [*], and obtain from such other Party [*] to the extent that it [*],
which [*], and (iii) [*], except in the context of a [*] pursuant to this
Section 12.4.2(b), [*] or otherwise [*] without [*] and an [*]. In any Third
Party IP Infringement Suit that includes claims involving jointly-owned
intellectual property, if the Parties are jointly directing and controlling the
defense, the Parties shall each [*] with the defense of such claims; and if a
non-named Party [*] pursuant to the preceding sentence of this Section
12.4.2(b), then such Party shall [*].
(c) Infringement Action Against a Party's Sublicensee. In the
event that a Third Party brings an action against a Party's sublicensee alleging
that the use of a Collaboration Mouse or the Exploitation of a Collaboration
Antibody or Collaboration Product, or any other activities arising out of the
exercise of license rights granted in this Agreement, infringes one or more
trademarks, patents or other intellectual property rights held by such Third
Party, then upon becoming aware of such action such Party shall promptly notify
the other Party thereof in writing, and, if any claims asserted against the
sublicensee relate to such other Party's Technology or Licensed Trademarks, or
to any KM Patent Rights, KM Know How, or KM Trademarks, shall make [*] in the
defense of claims.
(d) Infringement Action Involving an Invalidity Claim. If a
Third Party at any time asserts against a Party a claim that any of the Patent
Rights relating to the Technology or the KM Patent Rights is invalid or
otherwise unenforceable (an "Invalidity Claim"), whether as a defense to an
infringement action brought against the Third Party pursuant to Section 12.3.1
hereof, or in an action brought against a Party(ies) by the Third Party, in
addition to such Party's applicable obligations under Section 12.4.2(a) and
12.4.2(b) hereof, such Party shall [*], such Invalidity Claim to the extent that
it relates to [*]. The Party responsible for responding to such claim shall be
the Party against which such claim is asserted. [*] relating to the [*] or the
[*] without the [*], which [*].
(e) Cooperation. In the event that a Third Party institutes a
Third Party IP Infringement Suit against a Party or any of its Affiliates or any
other Trademark, patent or other infringement suit against a Party's sublicensee
(as more fully described in Section 12.4.2(c) hereof)
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-124-
during the Term of this Agreement, any Party that is not controlling the defense
shall, and shall [*], to [*] to [*] and [*] with the [*], and except as
otherwise provided in Section 12.4.2(b) hereof, [*].
12.4.3 Retained Rights. Nothing in this Section 12.4 shall prevent
either Party, at its own expense, from obtaining any license or other rights
from Third Parties it deems appropriate in order to permit the full and
unhindered exercise of its rights under this Agreement.
ARTICLE XIII
CONFIDENTIALITY
13.1 Disclosure and Use Restrictions. Except as provided herein, the
Parties agree that, during the Term and for [*] years after this Agreement's
expiration or termination pursuant to Article XIV hereof, each Party (and its
Affiliates) shall hold in strict confidence and shall not disclose to any Third
Party (other than employees, Affiliates, legal counsel, consultants, auditors
and advisors who, except in the case of legal counsel and auditors, are bound in
writing by substantially similar confidentiality obligations) any Confidential
Information of the other Party. During such period, a Party (and its Affiliates)
shall not use Confidential Information of the other Party or its Affiliates or
sublicensees for any purpose, except as expressly permitted herein.
13.2 Authorized Disclosure.
13.2.1 In General. A receiving Party may disclose Confidential
Information to the extent that such disclosure is:
(a) made in response to a valid subpoena or order of a court
of competent jurisdiction or other supra-national, federal, national, regional,
state, provincial, or local governmental or regulatory body of competent
jurisdiction;
(b) otherwise required by law, in the opinion of competent
legal counsel having expertise in the relevant field(s) to the receiving Party,
which shall be provided to the disclosing Party in the form of a written legal
opinion at least ten (10) business days or within such shorter time as may be
reasonable under the circumstances, but in any event prior to the receiving
Party's disclosure of the Confidential Information pursuant to this Section
13.2.1(b);
(c) made by the receiving Party to the regulatory authorities
as required in connection with any filing, application or request for Regulatory
Approval; provided, however, that reasonable measures shall be taken to assure
confidential treatment of such information; or
(d) made by the receiving Party to its financial
institutions, banks and other advisors, or to such Party's consultants and
advisors in connection with a proposed merger, acquisition, reorganization or
financing; provided, however, that any such disclosure be made subject to a
non-disclosure agreement with terms no less stringent than those provided in
this Article XIII;
and provided, however, that in the case of each of the exceptions set forth in
Section 13.2.1 (a)-(c) hereof, the receiving Party shall first have given notice
to the disclosing Party and given the disclosing Party a reasonable opportunity
to use diligent efforts to limit disclosure and obtain confidential treatment or
quash such order and to obtain a protective order requiring that the
Confidential Information and documents that are the subject of such order be
held in confidence by such court or agency or, if disclosed, be used only for
the purposes for which the order was issued; and provided further that if a
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-125-
disclosure order is not quashed or a protective order is not obtained, the
Confidential Information disclosed in response to such court, governmental
order, or governmental agency requirement shall be limited to that information
which is legally required to be disclosed in response to such court,
governmental agency, or governmental order.
13.2.2 With Consent. A receiving Party may disclose Confidential
Information to the extent that the disclosing Party has provided its prior
written consent to such disclosure. The Parties acknowledge and agree that in
the course of developing and commercializing the technologies licensed under
this Agreement, and in order for each Party to adequately advertise and promote
the technologies pursuant to Section 6.3.1 hereof, certain disclosures of
Confidential Information may be appropriate and each disclosing Party agrees [*]
to any request by the receiving Party to disclose such Confidential Information
as may be reasonable for such purposes.
13.2.3 Third Party [*]. A receiving Party may disclose Confidential
Information to a Third Party with which it is [*] or [*] a [*] or other [*]
relating to the [*], including, without limitation, a [*] governing a [*] and/or
[*], to the extent that such disclosure relates [*] and provided that such Third
Party has entered into a written non-disclosure/confidentiality agreement
whereby such Third Party is bound by confidentiality and non-use obligations
which are substantially similar to the ones to which the receiving Party is
bound hereunder. With respect to all other Confidential Information, neither
Party shall disclose any such Confidential Information to such Third Parties,
except in accordance with Sections 13.2.1 and 13.2.2 hereof.
13.3 Use of Name. Each Party (and its Affiliates) may use the name of the
other Party (or an abbreviation or adaptation thereof) (a) in connection with
announcements and other permitted disclosures relating to this Agreement and the
activities contemplated hereby; (b) as required by applicable law; (c) as
reasonably necessary for the exercise of rights granted to such Party pursuant
to Section 6.3.1 hereof; and (d) otherwise as agreed in writing by such other
Party.
13.4 Press Releases and Filings with the SEC. Neither Party (or its
Affiliates) shall issue a press release nor make any other public disclosure of
the terms of this Agreement without the prior approval of such press release or
public disclosure by the other Party hereto. Each Party shall submit any such
press release or public disclosure to the other Party, and such other Party
shall have [*] business days to review and approve any such press release or
public disclosure, which approval shall not be unreasonably withheld. If such
other Party does not respond within such [*] business day period, the press
release or public disclosure shall be deemed approved. If a Party obtains the
approval of the other Party to make a public disclosure pursuant to this Section
13.4 in connection with a filing with or other submission to the SEC or other
regulatory authority, or if public disclosure is otherwise deemed approved
pursuant to the preceding sentence of this Section 13.4, the Party that has
obtained (or is deemed to have obtained) approval to make a disclosure (and such
Party's Affiliates) shall be permitted to make subsequent public disclosures to
the SEC or other regulatory authorities containing statements that are
substantially similar to the statements contained in such previously permitted
disclosures, without seeking the prior approval of the other Party with respect
to such subsequent disclosures. In addition and notwithstanding the foregoing,
if a public disclosure of the terms of this Agreement is required by law, as
reasonably determined by counsel to the Party seeking to make a disclosure,
including without limitation in a filing with or other submission to the SEC, to
be made within a period of less than [*] business days from the date such Party
becomes subject to such legal disclosure requirement, and (a) such Party has
provided copies of the disclosure to the other Party as far in advance of such
filing or other disclosure as is reasonably practicable under the circumstances,
(b) such Party has promptly notified the other Party in writing of such
requirement and any respective timing constraints, and (c) such Party has given
the other Party a reasonable time under the circumstances from the date of
notice by such Party of the required
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-126-
disclosure to [*], request confidential treatment or approve such disclosure,
then such Party shall have the right to make such public disclosure at the time
and in the manner reasonably determined by its counsel to be required by law or
governmental regulation. Notwithstanding anything to the contrary herein, it is
hereby understood and agreed that if in each case set forth in this Section
13.4, the other Party [*] within the respective time periods or constraints
specified herein or within the respective notice, the Party seeking to make such
disclosure or its counsel, as the case may be, shall [*] (i) [*] and (ii) [*] to
[*] and/or obtain [*] to the extent [*].
13.5 Equitable Relief. Each Party acknowledges and agrees that breach of
any of the terms of this Article XIII would cause irreparable harm and damage to
the other and that such damage may not be ascertainable in money damages and
that as a result thereof the non-breaching Party would be entitled to seek from
a court as is contemplated by Section 17.2 hereof equitable or injunctive relief
restraining any breach or future violation of the terms contained herein by the
breaching Party without the necessity of proving actual damages. Such right to
equitable relief is in addition to whatever remedies either Party may be
entitled to as a matter of law or equity, including money damages, which other
remedies are subject to the provisions of this Agreement concerning the
resolution of Disputes.
13.6 Third Party Agreements. Each Project Agreement entered into after the
Effective Date by a Party and its Affiliate, Partner, or In-House Collaborator
(or potential Partner or In-House Collaborator) involving the use of
Collaboration Mice to research, develop, and/or commercialize Collaboration
Products shall include terms and conditions governing use and disclosure of
Confidential Information that are consistent in all material respects with the
definition of Confidential Information and the terms and conditions set forth in
Sections 13.1-13.4 hereof.
ARTICLE XIV
TERM AND TERMINATION
14.1 Term. This Agreement shall take effect upon the Effective Date and
shall continue in effect until December 31, 2014, unless terminated at an
earlier date in accordance with the terms and conditions set forth in this
Article XIV.
14.2 Termination of this Agreement for Material Breach. In the event of a
material breach by a Party of any of its representations, warranties, covenants
or other obligations under this Agreement, the non-breaching Party may deliver
to the breaching party a notice specifying the nature of the alleged breach, and
the breaching Party shall cure such breach within the Cure Period. If such
notice is delivered to the breaching Party and the material breach is not cured
during the Cure Period or, if such breach cannot be cured within such period,
the Party in breach does not commence actions to cure such breach within the
Cure Period and thereafter diligently continue such actions until cured, the
non-breaching Party shall be entitled, without prejudice to any of its other
rights and benefits conferred on it by this Agreement, and in addition to any
other remedies available to it by law or in equity, to terminate this Agreement
in its entirety. If the Party alleged to be in breach disputes the alleged
breach, it may exercise its right to invoke the dispute resolution procedures
set forth in Article XVII hereof. For purposes of clarification, the Parties
hereby acknowledge and agree that if [*] because a [*] exists in its favor, such
failure to perform shall [*] by such Party if a [*] pursuant to Section 8.11.4
hereof.
14.3 Consequences of Expiration or Termination.
14.3.1 Expiration. Except as provided in Sections 10.9.1 and 10.9.2
hereof, upon expiration of the full Term of this Agreement in accordance with
Section 14.1 (i.e., December 31, 2014)
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-127-
hereof (but not upon any earlier termination of this Agreement), the licenses
granted by a Party to the other Party hereunder shall be deemed [*], and the
licenses under Kirin Technology to Medarex, under the Medarex Technology to
Kirin, and under the KM-Mice, KM Patent Rights, and KM Know-How from one Party
to the other Party shall [*]. Following expiration of this Agreement, any
license subject to [*] hereof shall become [*].
14.3.2 Effect of Expiration or Termination on Sublicenses. Upon
expiration or termination of this Agreement pursuant to this Article XIV, any
sublicenses or other rights (including options) granted by a Party to a Third
Party pursuant to an agreement in effect at such expiration or termination date
with outstanding payment, performance, or other obligations shall, [*];
provided, however, that (a) such agreement was properly entered into consistent
with the terms and conditions of this Agreement and the sublicense was properly
granted; (b) the sublicensee is not then in breach of the agreement containing
the terms and conditions of the sublicense (or any Direct Sublicense Agreement);
and (c) all the restrictions and limitations of this Agreement apply to the
sublicensee under such agreement (and any Direct Sublicense Agreement) as though
this Agreement continued in effect.
14.3.3 Termination Pursuant to Section 14.2.
(a) Destruction of Mice, Mice Materials and Antibodies.
Within a reasonable time after termination of this Agreement in its entirety by
either Party pursuant to Section 14.2 hereof, each Party, at the request of the
other Party, shall destroy, in the case of Medarex as the destroying Party, all
Kirin Mice, Mice Materials derived therefrom and Antibodies and Antibody
Products obtained through the use of such mice, that are in Medarex's possession
as of the date of termination, and in the case of Kirin as the destroying Party,
all Medarex Mice, Mice Materials derived therefrom, and Antibodies and Antibody
Products obtained through the use of such mice, that are in Kirin's possession
as of the date of termination. Promptly following such destruction, an agent of
the destroying Party shall provide the other Party with written certification
thereof.
(b) No Liability. Neither Party shall incur any liability
whatsoever for any damage, loss or expenses of any kind suffered or incurred by
the other arising from or incident to any lawful termination of this Agreement
(or any part thereof) by such Party which complies with the terms of the
Agreement whether or not such Party is aware of any such damage, loss or
expenses.
14.4 Cure in Certain Instances. Notwithstanding anything else contained in
this Agreement, in the event that Medarex grants to Kirin a license (or a
sublicense) in Section 8.2 hereof or Medarex grants to a Kirin Affiliate, Kirin
Partner or Kirin In-House Collaborator a license (and/or a sublicense) in a
Direct Sublicense Agreement, which license (and/or sublicense) is [*] under the
Technology and/or Medarex's interest in the KM Patent Rights and/or KM Know-How
with respect to one or more antibody(ies) pursuant to an agreement [*], then for
purposes of Sections 8.11 and 14.2 hereof, the exercise by Medarex of [*] to [*]
for Kirin the [*] in the [*] under the [*] and [*] in the [*] and/or [*] in
accordance with Section [*] hereof with respect to the [*] in the [*] shall be
[*] of an [*] under this Agreement with respect thereto, [*], provided, however,
that this Section 14.4 shall not be construed to apply to any other breach of
Medarex's obligations hereunder, including, without limitation, breach by
Medarex of its obligations to (a) follow the procedures set forth in Section 2.3
hereof with respect to (i) the listing of Antigens on any Antigen Lists that
Medarex has an obligation to maintain pursuant to Section 2.1.1 hereof, or (ii)
the listing of Antibodies on the Antibody Sequence List; or (b) respond to (i)
Antigen Availability Inquiries pursuant to Section 3.3 hereof, or (ii) Antibody
Availability Inquiries
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-128-
pursuant to Section 3.5 hereof. For purposes of Section 8.2.7(e) hereof and this
Section 14.4, [*] shall be tested as compared to the [*] that is [*], in the
[*], nor a [*].
14.5 Rights in Bankruptcy.
14.5.1 Licenses of "Intellectual Property." The Parties acknowledge
and agree that all rights and licenses granted under or pursuant to this
Agreement by Medarex or Kirin are, and shall otherwise be deemed to be, licenses
of rights to "intellectual property" for purposes of the Bankruptcy Code,
including without limitation Section 101 thereof. The Parties agree that the
Parties, as licensees of such rights under this Agreement, may retain and may
fully exercise all of their rights and elections under the Bankruptcy Code
(including, without limitation, under Section 365(n) of the Bankruptcy Code) and
any rights and elections provided to licensees under the Japanese bankruptcy law
or other Japanese laws applicable to bankruptcies or insolvencies. Without
limitation of the foregoing, the Parties agree that, to the maximum extent
permitted by applicable law, in any bankruptcy proceeding commenced by or
against a Party under Japanese law, the non-debtor Party shall retain the
licenses and other rights granted to it in Article VIII hereof and may continue
to exercise such rights in accordance with the terms and conditions of this
Agreement, irrespective of whether the debtor Party elects to rescind this
Agreement pursuant to Article 59 of the Japanese bankruptcy law (or any
comparable provision of other Japanese laws applicable to bankruptcies or
insolvencies).
14.5.2 Embodiments of Intellectual Property. The non-debtor, licensee
Party shall have rights to embodiments of the intellectual property of the
debtor Party that is licensed to the non-debtor Party under this Agreement
("Embodiments of Intellectual Property"), as those rights are set forth in
Section 365(n) of the Bankruptcy Code (or any analogous provision of Japanese
law enacted from time to time, in the case of a bankruptcy or rehabilitation
proceeding in Japan).
14.5.3 Supplementary Agreements. The Parties acknowledge and agree
that each of the [*] Agreement and the [*] Agreement is and shall be deemed to
be an "agreement supplementary to" this Agreement, as that phrase is used in
Section 365(n) of the Bankruptcy Code.
14.5.4 Effect of Bankruptcy Filing. In the event that one Party
becomes a debtor in a case under the Bankruptcy Code (or becomes the subject of
a comparable proceeding under Japanese bankruptcy law or other Japanese laws
applicable to bankruptcies or insolvencies), then unless or until this Agreement
is rejected or deemed rejected pursuant to Section 365(n) of the Bankruptcy Code
(or, alternatively, unless or until this Agreement is rescinded pursuant to
Article 59 of the Japanese bankruptcy law or any comparable provision of other
Japanese laws applicable to bankruptcies or insolvencies), debtor Party, or its
trustee (or similar representative under Japanese law) shall, upon the written
request of the non-debtor Party, (a) either (i) perform this Agreement, or (ii)
provide to the non-debtor Party a complete and accurate duplicate of (or
complete access to) all Embodiments of Intellectual Property not in the
non-debtor Party's possession upon the commencement of any such bankruptcy
proceeding (or comparable Japanese proceeding), to the full extent required by
Section 365(n)(4) of the Bankruptcy Code or any analogous provision of Japanese
law; and (b) not interfere with the rights of the non-debtor Party as licensee
or any of its permitted sublicensees under this Agreement or any agreement
supplementary to this Agreement, to all intellectual property of the debtor
Party licensed to non-debtor Party under this Agreement.
14.5.5 Consequences of Rejection. In the event that one Party becomes
a debtor in a case under the Bankruptcy Code (or becomes the subject of a
comparable proceeding under Japanese bankruptcy law or other Japanese laws
applicable to bankruptcies or insolvencies) and such Party in its capacity as a
debtor in possession or through its trustee or in any other capacity or through
any other
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-129-
successor rejects this Agreement pursuant to Section 365 of the Bankruptcy Code
(or rescinds this Agreement pursuant to Article 59 of the Japanese bankruptcy
law or any comparable provision of other Japanese laws applicable to
bankruptcies or insolvencies), or in the event this Agreement is deemed rejected
under the Bankruptcy Code (or deemed rescinded under any such Japanese law)
because it is not capable of assumption and assignment under the Bankruptcy Code
(or of ratification under any such Japanese law), other than as a result of the
failure or refusal of the non-debtor Party to consent to the assumption (or
ratification) of this Agreement, then:
(a) the non-debtor Party in its capacity as a licensee of the
other Party's intellectual property, may:
(i) treat this Agreement as terminated; or
(ii) retain its rights hereunder and under the [*]
Agreement (including the right to enforce the exclusivity provisions of the
licenses granted in this Agreement) to the full extent permitted by Section
365(n) of the Bankruptcy Code (or any analogous provision of Japanese law
enacted from time to time, as applicable); and
(b) if the non-debtor Party elects to retain its rights
pursuant to Section 14.5.5(a)(ii) hereof, the non-debtor Party shall be deemed
to have requested of the debtor Party as debtor in possession (or of its trustee
(or similar representative under Japanese law), if any) that the same (i)
immediately provide and deliver to the non-debtor Party, all intellectual
property of the debtor Party licensed to the non-debtor Party under this
Agreement and all Embodiments of the Intellectual Property without further
notice or seeking relief from the automatic stay or other leave from any court,
if not previously delivered to the non-debtor Party; and (ii) not interfere with
the rights of the non-debtor Party as licensee or any of its permitted
sublicensees under this Agreement or any agreement supplementary to this
Agreement, to such intellectual property.
14.6 Accrued Rights; Surviving Obligations.
14.6.1 Accrued Rights. Termination or expiration of this Agreement
for any reason shall be without prejudice to any rights that shall have accrued
to the benefit of a Party prior to such termination or expiration. No provision
under this Article XIV shall be interpreted to limit any rights to any other
remedies a Party may have at law or in equity. Such termination or expiration
shall not relieve a Party from obligations that are expressly indicated to
survive the termination or expiration of this Agreement.
14.6.2 Survival. Articles XII, XIII, XV, XVII and XVIII, and Sections
2.6, 8.6.1(b), 8.6.2, 8.6.3, 8.7, 10.9.1, 10.9.2, 10.12, 14.3, 14.5, and 16.5
and this Section 14.6 of this Agreement shall survive expiration or termination
of this Agreement for any reason.
14.7 Notices Relating to Project Agreements for Kirin Partner Projects and
Kirin In-House Projects. The Parties acknowledge and agree that, by virtue of
the fact that Medarex will enter into Direct Sublicense Agreements with Kirin's
Partners and In-House Collaborators, which Direct Sublicense Agreements shall
contain cross-default provisions that permit Medarex to terminate such Direct
Sublicense Agreements in the event of defaults by such Partners or In-House
Collaborators under agreements entered into with Kirin if such defaults
adversely affect Medarex, Medarex has an interest in being kept informed by
Kirin of any such default. Kirin shall provide prompt written notice to Medarex
of any such default by a Kirin Partner or Kirin In-House Collaborator, setting
forth the nature of the
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-130-
default and the date on which the cure period expires, and thereafter shall
provide prompt subsequent written notice to Medarex indicating whether such
default was timely cured.
ARTICLE XV
INDEMNITY
15.1 Indemnification of Medarex. Kirin shall indemnify Medarex, its
Affiliates, and solely in such capacities, its directors, officers, employees,
and agents and defend and save each of them harmless, from and against any and
all Losses arising from or occurring as a result of (a) the breach by Kirin of
this Agreement, (b) the breach by Kirin of any of the Kirin In-License
Agreements, if any, (c) the gross negligence or willful misconduct on the part
of Kirin in performing Kirin's obligations under this Agreement, or (d) any
activities of Kirin, its Affiliates, Partners or In-House Collaborators relating
to any Collaboration Mice or the Exploitation of any Collaboration Antibodies or
Collaboration Products, except (i) to the extent such Losses arise from [*] of
[*] of [*] directly [*] of the [*] of (1) [*], (2) the [*] (to the extent that
such [*] the incorporation of [*]), or (3) the [*] described in clause (i)(1) or
(i)(2) of this Section 15.1 and/or [*], and (ii) for those Losses for which
Medarex has an obligation to indemnify Kirin pursuant to Section 15.2.1 hereof,
as to which Losses under clause (ii) of this Section each Party shall indemnify
the other to the extent of their respective liability for the Losses, provided,
however, that Kirin shall not be obligated to indemnify for Losses that arise as
a result of gross negligence or willful misconduct on the part of Medarex or any
of its Affiliates, Partners or In-House Collaborators.
15.2 Indemnification of Kirin.
15.2.1 Subject to Section 15.2.2 hereof, Medarex shall indemnify
Kirin, its Affiliates, and solely in such capacities, its directors, officers,
employees, agents and defend and save each of them harmless, from and against
any and all Losses arising from or occurring as a result of (a) the breach by
Medarex of this Agreement, (b) the breach by Medarex of any of the Medarex
In-License Agreements, (c) the gross negligence or willful misconduct on the
part of Medarex in performing Medarex's obligations under this Agreement, or (d)
any activities of Medarex, its Affiliates, Partners or In-House Collaborators
relating to any Collaboration Mice or the Exploitation of any Collaboration
Antibodies or Collaboration Products, except (i) to the extent such Losses arise
from [*] of [*] of [*] directly [*] of the [*] of (1) [*], (2) the [*] (to the
extent that such [*] the incorporation of [*]), or (3) the [*] described in
clause (i)(1) or (i)(2) of this Section 15.2.1 and/or [*], and (ii) for those
Losses for which Kirin has an obligation to indemnify Medarex pursuant to
Section 15.1 hereof, as to which Losses under clause (ii) of this Section each
Party shall indemnify the other to the extent of their respective liability for
the Losses, provided, however, that Medarex shall not be obligated to indemnify
for Losses that arise as a result of gross negligence or willful misconduct on
the part of Kirin or any of its Affiliates, Partners or In-House Collaborators.
15.2.2 Notwithstanding anything contained in Section 15.2.1 hereof,
or the other terms and conditions of this Agreement, Medarex shall not be
required to indemnify Kirin or any of its Affiliates, Partners, In-House
Collaborators, directors, officers, employees or agents, or defend and save any
of them harmless, from and against any Losses arising from or occurring as a
result of Medarex's granting to Kirin a license (and/or a sublicense) in Section
8.2 hereof or Medarex granting to a Kirin Affiliate, Kirin Partner or Kirin
In-House Collaborator a license (and/or a sublicense) in a Direct Sublicense
Agreement, which license (and/or sublicense) is [*] under the Technology and/or
the KM Patent Rights and/or KM Know-How with respect to one or more
antibody(ies) pursuant to an agreement [*]; provided, however, that this Section
15.2.2 shall not apply to the extent that the above-described Losses arise from
or occur as a result of any breach by Medarex from and after the Effective Date
of its
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-131-
obligations to (a) follow the procedures set forth in Section 2.3 hereof with
respect to (i) the listing of Antigens on any Antigen Lists that Medarex has an
obligation to maintain pursuant to Section 2.1.1 hereof, or (ii) the listing of
Antibodies on the Antibody Sequence List; or (b) respond to (i) Antigen
Availability Inquiries pursuant to Section 3.3 hereof, or (ii) Antibody
Availability Inquiries pursuant to Section 3.5 hereof.
15.3 Indemnification Procedure.
15.3.1 Notice of Claim. When requesting indemnification under Section
15.1 or Section 15.2.1 hereof, the Indemnified Party shall promptly give the
Indemnifying Party an Indemnification Claim Notice, but in no event shall the
Indemnifying Party be liable for any Losses that result from any delay in
providing such notice. The Indemnified Party shall furnish promptly to the
Indemnifying Party copies of all papers and official documents received in
respect of any Losses. All indemnification claims in respect of an Indemnified
Party, or their respective directors, officers, employees, and agents shall be
made solely by such Indemnified Party.
15.3.2 Third Party Claims. The obligations of an Indemnifying Party
under this Article XV with respect to Losses arising from Third Party Claims
shall be governed by and be contingent upon the following additional terms and
conditions:
(a) Control of Defense. At its option, the Indemnifying Party
may assume the defense of any Third Party Claim by giving notice to the
Indemnified Party within [*] days after the Indemnifying Party's receipt
of an Indemnification Claim Notice. The assumption of the defense of a Third
Party Claim by the Indemnifying Party shall not be construed as an
acknowledgment that the Indemnifying Party is liable to indemnify any
Indemnified Party in respect of the Third Party Claim, nor shall it constitute a
waiver by the Indemnifying Party of any defenses it may assert against any
Indemnified Party's claim for indemnification. Upon assuming the defense of a
Third Party Claim, the Indemnifying Party may appoint as lead counsel in the
defense of the Third Party Claim any legal counsel selected by the Indemnifying
Party and, in the event of a conflict of interest between the Indemnifying and
Indemnified Parties with respect to such Third Party Claim, the Indemnified
Party shall have the right to appoint its own single counsel, acceptable to and
at the expense of the Indemnifying Party, to participate in the resolution of
such Third Party Claim; provided, however, that the Indemnified Party shall only
be permitted to retain separate counsel pursuant to this Section 15.3.2(a) if,
upon request of the Indemnifying Party, it first delivers to the Indemnifying
Party a legal opinion prepared by counsel acceptable to the Indemnifying Party,
which counsel shall not be affiliated or have been previously retained by the
Indemnified Party or any of its Affiliates, concluding that there is a conflict
of interest between the Parties sufficient to warrant the retention of separate
counsel. In the event the Indemnifying Party assumes the defense of a Third
Party Claim, the Indemnified Party shall immediately deliver to the Indemnifying
Party all original notices and documents (including court papers) received by
any Indemnified Party in connection with the Third Party Claim. Should the
Indemnifying Party assume the defense of a Third Party Claim and thereafter
determine it is not obligated to provide indemnification hereunder, it shall
promptly so notify the Indemnified Party. Any such notice shall include a
well-reasoned statement explaining why indemnification in respect of such Third
Party Claim is not required hereunder. After providing such notice the
Indemnifying Party shall not be liable to the Indemnified Party or any other
Indemnified Party for any legal expenses subsequently incurred by such
Indemnified Party in connection with the analysis, defense, or settlement of the
Third Party Claim, unless it is later determined that indemnification in respect
of such Third Party Claim was indeed required. In the event that it is
ultimately determined that the Indemnifying Party is not obligated to indemnify,
defend, or hold harmless an Indemnified Party from and against the Third Party
Claim, the Indemnified Party shall reimburse the Indemnifying Party for any and
all costs and expenses (including reasonable attorneys' fees
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-132-
and costs of suit) and any Losses incurred by the Indemnifying Party in its
defense of the Third Party Claim with respect to such Indemnified Party. If,
however, it is ultimately determined that the Indemnifying Party was obligated
to provide indemnification hereunder, the Indemnifying Party shall be
responsible for prompt payment of any and all costs and expenses (including
reasonable attorneys' fees and costs of suit) and any Losses incurred by the
Indemnified Party in its defense of the Third Party Claim.
(b) Right to Participate in Defense. Without limiting Section
15.3.2(a) hereof, any Indemnified Party shall be entitled to participate in, but
not control, the defense of such Third Party Claim and to employ counsel of its
choice for such purpose; provided, however, that such employment shall be at the
Indemnified Party's own expense unless (i) the employment thereof has been
specifically authorized by the Indemnifying Party in writing, or (ii) the
Indemnifying Party has failed to assume the defense and employ counsel in
accordance with Section 15.3.2(a) hereof (in which case the Indemnified Party
shall control the defense).
(c) Settlement. With respect to any Third Party Claims where
the Indemnifying Party has assumed the defense thereof in accordance with
Section 15.3.2(a) hereof, the Indemnifying Party shall have the sole authority
to consent to the entry of any judgment, enter into any settlement, or otherwise
dispose of such Loss, provided it obtains the prior written consent of the
Indemnified Party (which consent shall not be unreasonably withheld). The
Indemnifying Party shall not be liable for any settlement or other disposition
of a Loss by an Indemnified Party that is reached without the written consent of
the Indemnifying Party. Regardless of whether the Indemnifying Party chooses to
defend or prosecute any Third Party Claim, no Indemnified Party shall admit any
liability with respect to, or settle, compromise or discharge, any Third Party
Claim without the prior written consent of the Indemnifying Party.
(d) Cooperation. In the event that the Indemnifying Party
assumes the defense or prosecution of a Third Party Claim, the Indemnified Party
shall, and shall cause each other Indemnified Party to, cooperate in the defense
or prosecution thereof and shall furnish such records, information and
testimony, provide such witnesses and attend such conferences, discovery
proceedings, hearings, trials and appeals as may be reasonably requested in
connection therewith. Such cooperation shall include access during normal
business hours afforded to the Indemnifying Party to, and reasonable retention
by the Indemnified Party of, records and information that are reasonably
relevant to such Third Party Claim, and making Indemnified Parties and other
employees and agents available on a mutually convenient basis to provide
additional information and explanation of any material provided hereunder, and
the Indemnifying Party shall reimburse the Indemnified Party for all its
reasonable out-of-pocket expenses in connection therewith.
(e) Expenses. Except as provided above, the costs and expenses,
including reasonable fees and disbursements of counsel, incurred by the
Indemnified Party in connection with any claim shall be reimbursed on a Calendar
Quarter basis by the Indemnifying Party, without prejudice to the Indemnifying
Party's right to contest the Indemnified Party's right to indemnification and
subject to refund in the event the Indemnifying Party is ultimately held not to
be obligated to indemnify the Indemnified Party.
15.4 Insurance. Each Party shall have and maintain (a) commercial general
liability insurance (in each country, as is customary on a country-by-country
basis) at all times during the Term, solely in the territories in which it has
or maintains substantial operations, which, for the avoidance of doubt, [*] for
the purposes of [*], and [*] for the purposes of [*], and (b) [*] (or with
respect to [*]) commencing no later than the [*] by such Party for [*], in the
[*], in such [*] as such Party [*] and as is
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-133-
[*]. Maintenance of such insurance coverage shall not relieve either Party of
any responsibility under this Agreement for damages in excess of insurance
coverage limits or otherwise.
ARTICLE XVI
REPRESENTATIONS AND WARRANTIES
16.1 Representations, Warranties and Covenants. Each Party hereby
represents and warrants to the other Party as of the Effective Date and further
covenants to the other Party, as follows:
16.1.1 Such Party (a) has the power and authority and the legal
right to enter into this Agreement, perform its obligations hereunder, and,
subject to the terms and conditions set forth herein, grant the rights,
licenses, sublicenses and further sublicenses provided for herein, and (b) has
taken all necessary action on its part required to authorize the execution and
delivery of this Agreement and the performance of its obligations hereunder.
This Agreement has been duly executed and delivered on behalf of such Party and
constitutes a legal, valid and binding obligation of such Party and is
enforceable against it in accordance with its terms subject to the effects of
bankruptcy, insolvency or other laws of general application affecting the
enforcement of creditor rights and judicial principles affecting the
availability of specific performance and general principles of equity, whether
enforceability is considered a proceeding at law or equity.
16.1.2 There is no pending litigation in which such Party or any of
its Affiliates is named as a party and has been served with process and, to the
knowledge of the officers of such Party, there is no threatened litigation
against such Party or any of its Affiliates (and such Party has not received any
communication) that alleges that such Party's activities related to this
Agreement have violated, or that by conducting the activities as contemplated
herein such Party would violate, any of the intellectual property rights of any
other Person.
16.1.3 Except in relation to the Parties' ▇▇▇▇-▇▇▇▇▇-▇▇▇▇▇▇ filings,
as provided in Section 8.9 hereof, all necessary consents, approvals and
authorizations of all regulatory and governmental authorities and other Persons
required to be obtained by such Party in connection with the execution and
delivery of this Agreement and the performance of its obligations hereunder have
been obtained.
16.1.4 The execution and delivery of this Agreement and the
performance of such Party's obligations hereunder (a) do not conflict with or
violate any requirement of applicable law or regulation or any provision of the
articles of incorporation, bylaws, limited partnership agreement or any similar
instrument of such Party, as applicable, in any material way, and (b) [*] do not
in any material way conflict with, violate, or breach or constitute a default or
require any consent under, any contractual obligation or court or administrative
order by which such Party is bound.
16.1.5 At no time shall such Party or its Affiliates, directly or
indirectly, expressly or by implication, by action or omission or otherwise (a)
assign, transfer, convey or otherwise encumber any right, title or interest in
or to its Patent Rights or Know-How, (b) grant any license or other right, title
or interest in or to its Patent Rights or Know-How, or (c) agree to or otherwise
become bound by any covenant not to ▇▇▇ for any infringement, misuse or other
action or inaction with respect to its Patent Rights or Know-How, in each case
that is inconsistent with the grants, assignments, and other rights reserved to
the other Party under this Agreement.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-134-
16.1.6 Each Party has or shall obtain from its employees and agents,
and from each of its Affiliates and such Affiliates' employees and agents, all
rights to Information and Inventions that relate to the Technology and KM Patent
Rights, such that each Party shall receive from the other Party the licenses and
other rights granted to such Party hereunder, without payment obligations,
except those set forth in Article X.
16.1.7 Each Party has or shall obtain from its Affiliates all rights
to Technology and to the KM Patent Rights necessary in order to meet its
obligations hereunder.
16.1.8 Each Party shall cause its respective Affiliates to take any
and all actions required by the terms of this Agreement to be taken by such
Affiliates, and to refrain from taking any and all actions which such Affiliates
are prohibited from taking by the terms of this Agreement.
16.1.9 Each Party shall comply with applicable law in exercising any
of the licenses or other rights granted to such Party by the other Party in this
Agreement.
16.2 Additional Representations, Warranties and Covenants of
Medarex. Medarex represents and warrants to Kirin as of the Effective Date and
further covenants to Kirin that:
16.2.1 Medarex, Inc. is a corporation duly organized, validly
existing and in good standing under the laws of the State of New Jersey and
GenPharm International, Inc. is a corporation duly organized, validly existing
and in good standing under the laws of the State of California. Each of Medarex,
Inc. and GenPharm International, Inc. has full corporate power and authority and
the legal right to own and operate its property and assets and to carry on its
business as it is now being conducted and as it is contemplated to be conducted
by this Agreement.
16.2.2 Exhibit C contains a true, correct and complete list of all
of the agreements entered into between Medarex and a Third Party entered into
prior to December 27, 1999, pursuant to which Medarex (a) has received a license
from such Third Party with respect to Patent Rights that qualify as "Medarex
Patent Rights" for purposes of this Agreement, and (b) Medarex is permitted to
grant sublicense rights to Kirin with respect to at least some of the rights
granted to Medarex in such agreements. Medarex has provided to Kirin true,
correct and complete copies of the Medarex In-License Agreements [*], which
agreement [*] hereby [*] provided in Section [*] hereof), to the extent that
Medarex has reasonably determined that it is permitted by the terms of each such
agreement to provide such copies to Kirin. As of the Effective Date, Medarex is
[*] During the Term of this Agreement, Medarex shall [*] and it shall [*],
without [*], take [*] any such [*] if such [*] would [*] under this Agreement or
otherwise [*]. If at any time during the Term of this Agreement it is determined
that any agreement entered into by Medarex and a Third Party should have been
but was not included on Exhibit C as of the Effective Date, the Parties shall
amend Exhibit C to include such agreement.
16.2.3 During the period beginning on December 27, 1999, and ending
on the Effective Date, Medarex has [*] the [*] to [*] any Antibody or Antibody
Product, or [*] to [*] to [*] any Antibody or Antibody Product, except to the
extent such [*] was (a) [*] the Agreement on Essential Terms for Collaboration,
or (b) [*] and [*] the writing exchanged by the Parties pursuant to Section
8.8.1 hereof. During the Term of this Agreement, Medarex shall [*] to [*] any
Antibody or Antibody Product, or [*] to [*] any Antibody or Antibody Product,
except to the extent such [*].
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-135-
16.3 Additional Representations, Warranties and Covenants of Kirin. Kirin
represents and warrants to Medarex as of the Effective Date and further
covenants to Medarex that:
16.3.1 Kirin is a corporation duly organized, validly existing and
in good standing under the laws of Japan, and has full corporate power and
authority and the legal right to own and operate its property and assets and to
carry on its business as it is now being conducted and as it is contemplated to
be conducted by this Agreement.
16.3.2 As reflected in Exhibit D attached hereto, Kirin has not
entered into [*], pursuant to which Kirin (a) has received a license from such
Third Party with respect to Patent Rights that qualify as "Kirin Patent Rights"
for purposes of this Agreement, and (ii) is permitted to grant sublicense rights
with respect to at least some of the rights granted to Kirin in such agreements.
If at any time during the Term of this Agreement it is determined that any
agreement entered into by Kirin and a Third Party should have been but was not
included on Exhibit D, the Parties shall amend Exhibit D to include such
agreement. With respect to any such agreement added to Exhibit D, if any, Kirin
shall provide to Medarex a true, complete and correct copy of such Kirin
In-License Agreement to the extent that Kirin has determined, [*] During the
Term of this Agreement, Kirin shall [*], if any, and it shall [*] take [*] if
such [*] would [*] under this Agreement or otherwise [*].
16.3.3 During the period beginning on December 27, 1999, and ending
on the Effective Date, Kirin has [*] the [*] to [*] any Antibody or Antibody
Product, or [*] the [*] to [*] any Antibody or Antibody Product, except to the
extent such [*]. During the Term of this Agreement, Kirin shall [*] the [*] to
[*] any Antibody or Antibody Product, or [*] the [*] to [*] any Antibody or
Antibody Product, except to the extent such [*] is [*].
16.4 Knowledge; Officers. For the purposes of this Article XVI, (a)
"knowledge of" a Party shall mean [*], and (b) "officers" shall mean, in the
case of Medarex, persons in the positions of vice president, senior vice
president, president and chief executive officer, and in the case of Kirin,
person in the positions of president and vice president of the Pharmaceutical
Division of Kirin.
16.5 Limitation of Warranty. EXCEPT FOR THE EXPRESS WARRANTIES SET FORTH
IN THIS AGREEMENT, KIRIN AND MEDAREX GRANT NO WARRANTIES, EXPRESS OR IMPLIED,
EITHER IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE; AND ANY MICE,
MICE MATERIALS, COLLABORATION ANTIBODIES AND ANTIBODY MATERIALS RELATING THERETO
SUPPLIED TO A PARTY, ITS AFFILIATES, PARTNERS OR IN-HOUSE COLLABORATORS PURSUANT
TO THIS AGREEMENT ARE SUPPLIED "AS IS", AND KIRIN AND MEDAREX EACH SPECIFICALLY
DISCLAIM ANY OTHER WARRANTIES, INCLUDING WITHOUT LIMITATION ANY IMPLIED WARRANTY
OF QUALITY, WARRANTY OF MERCHANTABILITY OR WARRANTY OF FITNESS FOR A PARTICULAR
PURPOSE OR ANY WARRANTY AS TO THE VALIDITY OF ANY PATENT OR THE NON-INFRINGEMENT
OF ANY INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-136-
ARTICLE XVII
DISPUTE RESOLUTION
17.1 General. If a Dispute arises between the Parties in any context other
than with regard to whether Rights Preserving Excuse have been triggered or
whether a proposed Cure is adequate, which are governed by Section 8.11, the
Parties shall use the following procedure in good faith prior to either Party
pursuing other available judicial or nonjudicial remedies:
17.1.1 Meeting. A meeting shall be held between the Parties within
[*] days after either Party gives the other Party a Dispute Notice. The meeting
shall be attended by a representative of each Party having decision-making
authority regarding the Dispute (subject to Board of Directors or equivalent
approval, if required), who shall attempt in good faith to negotiate a
resolution of the Dispute.
17.1.2 Arbitration. If, within [*] days after the Dispute Notice, the
Parties have not succeeded in negotiating a written resolution of the Dispute,
and a Party desires to pursue further resolution of the Dispute, such Party
shall refer the Dispute to [*] in accordance with the Commercial Dispute
Resolution Procedures of the American Arbitration Association ("AAA"), and at
the election of either Party or an arbitrator in appropriate instances, the
optional AAA rules for "Large, Complex Commercial Disputes" (except that with
respect to any such rules specific provisions of this Article XVII hereof shall
override inconsistent provisions of such rules) to be held in San Diego,
California. The Parties shall promptly negotiate in good faith to appoint an
Expert. If the Parties cannot agree on the appointment of an Expert to serve as
arbitrator within [*] days after receipt of a demand for arbitration, each Party
shall appoint one Expert to serve as an arbitrator, and the two arbitrators
shall appoint a third Expert to serve as arbitrator. If the Party-appointed
arbitrators cannot agree on the third Expert to serve as arbitrator, the third
arbitrator shall be appointed in accordance with the Commercial Arbitration
Rules of the American Arbitration Association. The arbitrator(s) shall hold a
hearing to resolve the issues within [*] days after selection.
17.1.3 [*] The Parties acknowledge and agree that, to the extent
consistent with expedited process and not resulting in any significant delay of
the arbitration, each Party will have the right to [*], including, without
limitation, [*] by the other Party to [*] in the arbitration proceeding, and
that (subject to this Section 17.1.3) the [*] shall be [*] of the matters in
dispute as [*] by the appointed arbitrators.
17.1.4 [*] The Parties shall participate in good faith in the
arbitration to its conclusion. The recommendation of the arbitrator(s) shall be
[*] upon the Parties, except that [*]. A judgment on the arbitrator's
disposition may be entered in any court having jurisdiction over the Parties.
17.2 Interim Relief. Notwithstanding anything herein to the contrary,
nothing in this Section shall preclude either Party from seeking interim or
provisional relief from a court located in the State of California, including a
temporary restraining order, preliminary injunction or other interim equitable
relief concerning a Dispute, either prior to or during the arbitration, if
necessary to protect the interests of such Party in the event of certain
breaches which will result in irreparable and continuing damage to the other
Party for which there will be no adequate remedy at law.
17.3 Language. The arbitration proceedings and all written evidence shall
be in the English language. Any written evidence originally in a language other
than English shall be submitted in English translation accompanied by the
original or a true copy thereof. Any recommended resolution provided by
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-137-
the arbitrator(s), and any explanation of the reasons for such recommended
resolution, shall be in the English language.
17.4 Covenant Relating to "WHEREAS" Clauses. Medarex and Kirin each
covenants to the other that, in the event of any litigation, arbitration or
other dispute resolution proceeding to determine claims by Medarex or by Kirin
(or any of either Party's Affiliates, successors or assigns) that the other
Party's Exploitation of the Kirin Mice, Kirin Technology, Medarex Mice, Medarex
Technology, KM-Mice, KM Patent Rights, and/or KM Know-How would infringe patent
or other intellectual property rights of either Medarex or Kirin (or any of
either Party's Affiliates, successors or assigns), neither Party (nor any of
either Party's Affiliates, successors or assigns) will make reference to the
fifth or sixth "WHEREAS" clause of this Agreement, or the drafting history of
those clauses, as evidence or support for any contention or determination that
either Medarex or Kirin (or any of either Party's Affiliates, successors or
assigns) has made any concession concerning the validity, weight, or
substantiality of such claims, or any concession that such claims have a
reasonable basis.
ARTICLE XVIII
MISCELLANEOUS
18.1 Force Majeure. Neither Party shall be held liable or responsible to
the other Party or be deemed to have defaulted under or breached this Agreement
for failure or delay in fulfilling or performing any term of this Agreement when
such failure or delay is caused by or results from causes beyond the reasonable
control of the non-performing Party, including earthquakes, fires, floods,
embargoes, shortages, epidemics, quarantines, war, acts of war (whether war be
declared or not), insurrections, riots, civil commotion, strikes, lockouts or
other labor disturbances, acts of God or acts, omissions or delays in acting by
any governmental authority. The non-performing Party shall notify the other
Party of such force majeure within [*] business days after such occurrence by
giving written notice to the other Party stating the nature of the event, its
anticipated duration, and any action being taken to avoid or minimize its
effect. The suspension of performance shall be of no greater scope and no longer
duration than is necessary and the non-performing Party shall use [*] to remedy
its inability to perform; provided, however, that in the event the suspension of
performance continues for [*] days after the date of the occurrence, and such
failure to perform would constitute a material breach of this Agreement in the
absence of such force majeure, the performing Party may terminate this Agreement
pursuant to Section 14.2 hereof by written notice to the other Party.
18.2 Governing Law. This Agreement is to be governed by and construed in
accordance with the laws of [*], without reference to the rules of conflict of
laws thereof.
18.3 Notices.
18.3.1 Except as otherwise provided in Sections 3.3.1(d) and 3.5.2
hereof, with respect to which Sections the method and address for delivery of
notices, inquiries or communications required pursuant to such Sections shall be
governed by the terms of such Sections, all notices, inquiries and other
communications that are required or permitted to be made by a Party to the other
Party under Articles II, III, IV, V, VI and VII hereof shall be made in writing
in the English language and (i) delivered electronically via email to the two
(2) addressees listed below (and in the case of delivery by email hereunder,
confirmed by a copy sent by internationally-recognized overnight courier
addressed as set forth below within one (1) day of dispatching the emails), or
(ii) sent by internationally-recognized overnight courier, addressed as follows:
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-138-
If to Kirin, emails to:
[*] and [*]
and by courier to:
Pharmaceutical Division
Kirin Brewery Co., Ltd.
▇▇-▇, ▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇, ▇▇▇▇▇, ▇▇▇-▇▇▇▇
▇▇▇▇▇
Attention: Vice President, Planning Department
If to Medarex, emails to:
[*] and [*]
and by courier to:
Medarex, Inc.
▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇ ▇▇▇
▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇-▇▇▇▇
Attention: General Counsel
Telecopier: (▇▇▇) ▇▇▇-▇▇▇▇
or to such other address as the Party to which notice is to be given may have
furnished to the other Party in writing in accordance herewith. Any such notice,
inquiry or other communication shall be deemed to have been given (i) if sent by
email, the earliest date and time at which it is received by both addressees
listed above for Medarex or Kirin, as the case may be, or (ii) if sent by
internationally-recognized overnight courier without prior email receipt by both
addressees listed above for Medarex or Kirin, as the case may be, the date and
time of receipt, which receipt shall be evidenced by a written acknowledgment of
receipt signed by such Party.
18.3.2 Except as otherwise provided in Section 18.3.1 hereof, all
notices or other communications that are required or permitted hereunder shall
be in writing in the English language and delivered personally, sent by
facsimile (and promptly confirmed by a copy sent by personal delivery,
registered or certified mail or overnight courier as provided herein), sent by
internationally-recognized overnight courier or sent by registered or certified
mail, postage prepaid, return receipt requested, addressed as follows:
If to Kirin, to:
Pharmaceutical Division
Kirin Brewery Co., Ltd.
▇▇-▇, ▇▇▇▇▇▇▇▇ ▇-▇▇▇▇▇, ▇▇▇▇▇▇▇-▇▇, ▇▇▇▇▇, ▇▇▇-▇▇▇▇
▇▇▇▇▇
Attention: President
Telecopier: ▇▇▇-▇-▇▇▇▇-▇▇▇▇
with copies to:
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-139-
Gemini Science, Inc.
▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇
▇▇ ▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Attention: President
Telecopier: (▇▇▇) ▇▇▇-▇▇▇▇
and
▇▇▇▇▇▇▇▇ Chance ▇▇▇▇▇▇ & ▇▇▇▇▇ LLP
▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇
▇▇▇ ▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇
Attention: ▇▇▇▇▇ ▇▇▇▇▇▇, Esq.
Telecopier: (▇▇▇) ▇▇▇-▇▇▇▇
If to Medarex, to:
Medarex, Inc.
▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇ ▇▇▇
▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇-▇▇▇▇
Attention: President
Telecopier: (▇▇▇) ▇▇▇-▇▇▇▇
with copies to:
Medarex, Inc.
▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇ ▇▇▇
▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇-▇▇▇▇
Attention: General Counsel
Telecopier: (▇▇▇) ▇▇▇-▇▇▇▇
and
▇▇▇▇▇▇▇▇▇ & ▇▇▇▇▇▇▇
▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇
Attention: ▇▇▇▇▇ ▇. ▇▇▇▇▇▇, Esq.
Telecopier: (▇▇▇) ▇▇▇-▇▇▇▇
or to such other address as the Party to which notice is to be given may have
furnished to the other Party in writing in accordance herewith. Any such
communication shall be deemed to have been given (a) when delivered, if
personally delivered or sent by facsimile on a business day, (b) on the [*]
business day after dispatch, if sent by internationally-recognized overnight
courier, and (c) on the [*] business day following the date of mailing, if sent
by mail. It is understood and agreed that this Section 18.3.2 hereof is not
intended to govern the day-to-day business communications necessary between the
Parties in performing their duties, in due course, under the terms of this
Agreement; provided, however that those communications cannot constitute or
excuse the need for the formal notices required by the Agreement.
18.3.3 Each notice, inquiry or other communication that is provided
by one Party to the other Party hereunder shall indicate the Section(s) of this
Agreement, if any, pursuant to which such notice, inquiry or other communication
is provided. For the avoidance of doubt, it is understood and
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-140-
agreed that in the event that a Party fails to include such Section reference(s)
in a notice, inquiry or other communication, or that a Party inadvertently
includes the incorrect Section reference(s), it shall not be a breach of such
Party's obligations under this Agreement.
18.4 Assignment. Except as otherwise provided in this Section 18.4,
neither this Agreement nor any interest hereunder shall be directly or
indirectly assigned or transferred (whether voluntarily, by operation of law, or
otherwise) by either Party without prior written consent of the other Party;
provided, however, that either Kirin or Medarex may assign or transfer this
Agreement without the consent of the other Party (a) to any Kirin Affiliate or
Medarex Affiliate, respectively, in accordance with this Agreement; or (b) to
any Third Party with which it may merge or consolidate, or which acquires all or
substantially all of its voting securities, or to which it may transfer all or
substantially all of its assets to which this Agreement relates if in any such
event (i) the assigning Party shall provide written notice to the other Party of
such assignment, (ii) in the event that the assigning Party is not the surviving
entity, it remains jointly and severally liable under this Agreement with the
relevant Kirin Affiliate, Medarex Affiliate or Third Party assignee, and (iii)
the relevant Kirin Affiliate or Medarex Affiliate assignee, Third Party assignee
or surviving entity assumes in writing all of the assigning Party's obligations
under this Agreement. For purposes of clarification with respect to subsection
(b) herein, a Third Party that merges or consolidates with a Party, or to which
a Party transfers all or substantially all of its assets to which this Agreement
relates, shall not be deemed to grant the other Party to this Agreement any
license to such Third Party's technology (as opposed to such Party's Technology)
in existence as of the effective date of such merger, consolidation or transfer,
unless such grant is made pursuant to a separate agreement. For purposes of this
Section 18.4, "voting securities" includes presently authorized voting
securities and securities into which such securities may hereafter be changed or
by which such voting securities may be exchanged after giving effect to the
terms of such change or exchange by way of cancellation and reissuance of voting
securities, reorganization, recapitalization, consolidation or otherwise. Any
purported assignment or transfer in violation of this Section shall be void.
18.5 Entire Agreement; Modifications. This Agreement, the exhibits hereto
and the cross-receipt delivered by each Party on the date of execution of this
Agreement (and all documents attached thereto) set forth and constitute the
entire agreement and understanding between the Parties with respect to the
subject matter hereof and all prior agreements, understandings, promises and
representations, whether written or oral, with respect thereto, including,
without limitation, the Agreement on Essential Terms for Collaboration and that
certain Agreement Governing Transfer of Materials between Medarex and Kirin
effective as of December 27, 1999, as amended, are superseded hereby. Each Party
confirms that it is not relying on any representations or warranties of the
other Party except as specifically set forth herein. No amendment, modification,
release or discharge hereof shall be binding upon the Parties unless in writing
and duly executed by authorized representatives of both Parties.
18.6 Severability. In the event that any provision of this Agreement
becomes or is declared by a court of competent jurisdiction to be illegal,
unenforceable or void, this Agreement shall be modified to provide the maximum
rights and benefits that are enforceable and otherwise shall continue in full
force and effect without said provision; provided that no such severability
shall be effective if it materially changes the economic or intellectual
property benefit of this Agreement to either Kirin or Medarex. In the event that
any provision of this Agreement becomes or is declared by a court of competent
jurisdiction to be illegal, unenforceable or void, and severability of such
provision would materially change the economic benefit of this Agreement to
either Kirin or Medarex, Kirin and Medarex shall modify such provision in
accordance with Section 18.5 hereof to obtain a legal, enforceable and valid
provision and provide an equivalent economic business or intellectual property
benefit to Kirin and Medarex that most nearly effects Kirin's and Medarex's
intent in entering into this Agreement.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-141-
18.7 Waiver. No waiver of any rights shall be effective unless assented to
in writing by the Party to be charged and the waiver of any breach or default
shall not constitute a waiver of any other right hereunder or any subsequent
breach or default.
18.8 Independent Contractors. It is expressly agreed that Medarex, on the
one hand, and Kirin, on the other hand, shall be independent contractors and
that the relationship between the two Parties shall not constitute a
partnership, joint venture or agency. Except otherwise expressly set forth
herein, neither Medarex, on the one hand, nor Kirin, on the other hand, shall
have the authority to make any statements, representations or commitments of any
kind, or to take any action, which shall be binding on the other, without the
prior written consent of the other Party to do so. All persons employed by a
Party shall be employees of such Party and not of the other Party and all costs
and obligations incurred by reason of any such employment shall be for the
account and expense of such Party.
18.9 Language. This Agreement is written and executed in the English
language. Any translation into any other language shall not be an official
version thereof, and in the event of any conflict in interpretation between the
English version and such translation, the English version shall control.
18.10 Descriptive Headings. The headings of the several sections of this
Agreement are intended for convenience of reference only and are not intended to
be a part of or to affect the meaning or interpretation of this Agreement.
18.11 Counterparts. This Agreement may be executed in counterparts, each of
which shall be deemed an original, but all of which together shall constitute
one and the same instrument.
18.12 No Third Party Rights. No provision of this Agreement shall be deemed
or construed in any way to result in the creation of any rights or obligations
in any other individual group, entity or organization not a party to this
Agreement.
18.13 Further Assurances. Each Party hereby agrees to duly execute and
deliver, or cause to be duly executed and delivered such further instruments and
do and cause to be done such further acts and things, including without
limitation, the filing of such additional assignments, agreements, documents and
instruments, that may be necessary or as the other Party hereto may at any time
and from time to time reasonably request in connection with this Agreement or to
carry out more effectively the provisions and purposes hereof or to better
assure and confirm unto such other Party its rights and remedies under this
Agreement.
18.14 Cumulative Remedies. Unless expressly set forth herein to the
contrary, all remedies set forth herein are cumulative and are in addition to
any and all remedies provided either Party at law or in equity.
18.15 Attorneys' Fees. In the event it becomes necessary for either Party
(or any of its Affiliates) to institute any legal action, including, without
limitation, an arbitration pursuant to Article XVII hereof, against the other
Party to enforce its rights hereunder, the prevailing Party shall be entitled to
recover from the non-prevailing Party reasonable attorneys' fees, court costs
or arbitration costs as applicable, and expenses related to such action.
[SIGNATURE PAGE TO FOLLOW]
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-142-
IN WITNESS WHEREOF, the undersigned are duly authorized to execute this
Agreement on behalf of Kirin and Medarex, as applicable.
KIRIN BREWERY CO., LTD. MEDAREX, INC.
By: /s/ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ By: /s/ ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇
▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, Ph.D. ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇, Ph.D., ▇.▇.
President, Pharmaceutical Division President and Chief Executive Officer
GENPHARM INTERNATIONAL, INC.
By: /s/ ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇, ▇.▇.
President and Chief Operating Officer
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
-143-
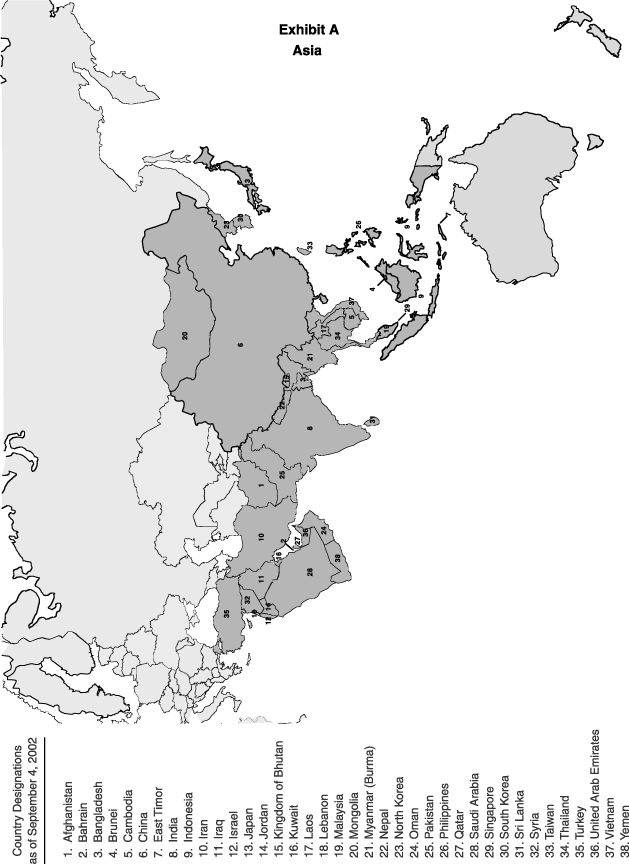
[*] = Certain confidential information contained
in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit A-1
EXHIBIT B
[*]
[*]
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit B-1
EXHIBIT C
Medarex In-License Agreements
1. Agreement between Abgenix, Inc., Cell Genesys, Inc., Japan Tobacco Inc. and
Xenotech L.P., on the one hand, and GenPharm International, Inc., on the
other hand, effective as of March 26, 1997.
2. Agreement between Medical Research Council and GenPharm International,
Inc., effective October 1, 1993, as amended on August 12, 1994 and on April
19, 2002.
3. Agreement between Pharming B.V. and GenPharm International, Inc., effective
as of July 1, 1996, as amended on November 29, 1996.
4. Agreement between DNX, Inc. and GenPharm International, Inc., effective as
of January 1, 1991.
5. Agreement between The University of Utah Research Foundation and GenPharm
International Inc., effective as of June 15, 1989.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit C-1
EXHIBIT D
Kirin In-License Agreements
None.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit D-1
EXHIBIT E
[*]
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit E-1
EXHIBIT F
[*]
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit F-1
EXHIBIT G
[*]
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit G-1
EXHIBIT H
[*]
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit H-1
EXHIBIT I
Database Description
The Target Database is a two-tier client server database, with Microsoft-SQL
2000 serving as the backend and Microsoft Access & Visual Basic components
making up the front end. Connection to the database is through native ADO using
the TCP/IP protocol. The design and implementation of the database was performed
using Microsoft best practices for designing 2-tier client server applications.
The Target Database is currently operating on a Dell 2450 server with a
redundant disk array (Level 5) with a total disc capacity of approximately 200
gigabytes.
The database security is encapsulated in the application and uses a trusted user
security model. The trusted user has been granted read, write and update access
rights to the database objects (views, triggers, tables, and stored procedures).
This user is used to form the initial connection to the database only from the
Medarex internal network. Once the initial connection is formed, users are
challenged for access credentials. The benefit of this security model is that
only the trusted user can connect to the database, no third party tools can be
used to circumvent application security. The password for the trusted user is
held by the database administrator.
The database has two types of auditing: activity logs and change logs. The
activity logs are used to track user activity in the database including what
users are viewing and reporting on and the date and time at which such activity
occurs. The change logs are available only to the database administrator and are
used to record any data associated with changes made to data records.
Backups of the Target Database are performed using Hewlett-Packard SureStore
tape libraries at the end of every twenty-four (24) hour period using a standard
Grandfather-Father-Son tape rotation system, which provides for a backup to be
taken offsite every week. Protection from site disaster is accounted for by
keeping backup tapes off site using a third party contractor.
The database server is under electronic key pad security in the Medarex,
Milpitas, California server room. The room is under a controlled environment and
has the benefit of a hidden camera on a motion sensor monitoring the room
continuously (twenty-four (24) hours per day, seven (7) days per week).
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit I-1
EXHIBIT J
Kirin Trademarks
----------------------------------------------------------------------------------------------------------------
Trademark Country The International Application No. Registration No.
Classification
----------------------------------------------------------------------------------------------------------------
T C Mouse Japan 31 4443885
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
T C Mouse Taiwan 31 939534
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
T C Mouse South Korea 31 491229
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
T C Mouse China 31 1590720
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
T C Mouse US 31 907867
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
T C Mouse Canada 31 1045600
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
T C Mouse Switzerland 31 474595
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
T C Mouse CTM(*) 31 001484724
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
T C Mouse New Zealand 31 607444
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
T C Mouse Australia 31 822044
TransChromoMouse
----------------------------------------------------------------------------------------------------------------
TC Mouse Unregistered
and no
applications
filed in any
country.
----------------------------------------------------------------------------------------------------------------
(*) CTM (= the Community trademark)
The current Member States are: Austria, Belgium, Denmark, Finland, France,
Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain,
Sweden, United Kingdom
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended
Exhibit J-1
EXHIBIT K
KM Trademarks
------------------------------------------------------------------------------------------------------
Trademark Country The International Application No. Registration No.
Classification
------------------------------------------------------------------------------------------------------
KM-Mouse Canada 31 1124419
------------------------------------------------------------------------------------------------------
KM-Mouse CTM(*) 31 0025485592
------------------------------------------------------------------------------------------------------
KM-Mouse US 31 Proposed
------------------------------------------------------------------------------------------------------
(*) CTM (= the Community trademark)
The current Member States are: Austria, Belgium, Denmark, Finland, France,
Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain,
Sweden, United Kingdom.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit K-1
EXHIBIT L
Medarex Trademarks
------------------------------------------------------------------------------------------------
Trademark Country The International Application No. Registration No.
Classification
------------------------------------------------------------------------------------------------
HuMAb-Mouse Canada 31 1106569
------------------------------------------------------------------------------------------------
HuMAb-Mouse CTM(*) 31, 42 002274314
------------------------------------------------------------------------------------------------
HuMAb-Mouse US - 74/490,454 2,234,442
------------------------------------------------------------------------------------------------
(*) CTM (= the Community trademark)
The current Member States are: Austria, Belgium, Denmark, Finland, France,
Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain,
Sweden, United Kingdom.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit L-1
EXHIBIT M
Pre-Existing Project Agreements Covering HuMAb License Projects and Partner
Projects
Kirin Agreement.
1. A Research and Commercialization Agreement between Kirin and Corixa
Corporation dated April 15, 2001.
Medarex Agreements.
------------------
1. A Research and Commercialization Agreement between Medarex, Inc., GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and
Amgen, Inc. dated September 21, 1999, as amended on November 16, 1999,
January 26, 2000, May 22, 2000, December 7, 2000, and December 27, 2000.
2. An Amended and Restated Material Transfer and Confidentiality and Option
Agreement between Medarex, Inc., GenPharm International, Inc., a wholly
owned subsidiary of Medarex, Inc., and ▇▇▇▇▇▇▇-▇▇▇▇▇ Squibb Company dated
June 11, 1998, as amended effective as of June 11, 2001.
3. A Research and Commercialization Agreement between Medarex, Inc, GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and B.
Twelve, Inc. dated January 15, 2001.
4. An Evaluation and Commercialization Agreement between Medarex, Inc, and
GenPharm International, Inc., a wholly owned subsidiary of Medarex, Inc.,
and Centocor, Inc. dated April 5, 2000.
5. A Research and Commercialization Agreement between GenPharm International,
Inc., a wholly owned subsidiary of Medarex, Inc., and ▇▇▇▇▇▇▇
Pharmaceutical, Inc. dated March 30, 2000.
6. A Research and Commercialization Agreement between Medarex, Inc., GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and ▇▇▇
Lilly and Company dated November 3, 2000.
7. A Research and Commercialization Agreement between GenPharm International,
Inc., a wholly owned subsidiary of Medarex, Inc., and EOS Biotechnology,
Inc. dated August 2, 1999, as amended and restated on March 17, 2000.
8. A Research and Commercialization Agreement between GenPharm International,
Inc., a wholly owned subsidiary of Medarex, Inc., Medarex, Inc., and
FibroGen, Inc. dated July 9, 1998, as amended on June 30, 2001.
9. A Research and Commercialization Agreement between Medarex, Inc., GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and
Genesto A/S dated August 13, 2001.
10. An Evaluation and Commercialization Agreement between GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., Medarex,
Inc., and Genmab A/S dated February 25, 1999, as amended on May 17, 1999,
May 19, 1999, and August 23, 2000.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit M-1
11. A Research and Commercialization Agreement between Medarex, Inc., GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and Human
Genome Sciences, Inc. dated May 23, 2001.
12. An Evaluation, Research and Commercialization Agreement between GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and
Immunex Corporation dated January 11, 1999.
13. An Evaluation and Commercialization Agreement between GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and
Leukosite, Inc. dated February 24, 1999.
14. A Research and Commercialization Agreement between Medarex, Inc., GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and
MedImmune, Inc. dated June 9, 2000.
15. An Evaluation, Research and Commercialization Agreement between GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and
Novartis Pharma AG dated November 6, 1998.
16. A Research and Commercialization Agreement between Medarex, Inc., GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and
NovImmune S.A. dated April 6, 2001.
17. A Research and Commercialization agreement between Medarex, Inc., GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and Novo
Nordisk A/S dated December 22, 2000.
18. A Research and Commercialization Agreement between Medarex, Inc., GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and
Oxford GlycoSciences (UK) Limited dated August 27, 2001.
19. A Research and Commercialization agreement between GenPharm International,
Inc., a wholly owned subsidiary of Medarex, Inc., Medarex, Inc., and Raven
Biotechnologies, Inc. dated March 24, 2000.
20. A Research and Commercialization Agreement between GenPharm International,
Inc., a wholly owned subsidiary of Medarex, Inc., Medarex, Inc., and
Schering AG dated February 9, 1998, as amended on May 11, 1999.
21. A Research and Commercialization Agreement between Medarex, Inc., GenPharm
International, Inc., a wholly owned subsidiary of Medarex, Inc., and
Schering-Plough Ltd. dated March 26, 2001.
22. A Research and Commercialization Agreement between GenPharm International,
Inc., a wholly owned subsidiary of Medarex, Inc., and ZymoGenetics, Inc.
dated October 6, 2000.
[*]=Certain confidential information contained in this document, marked by
brackets, has been omitted and filed separately with the Securities and Exchange
Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as
amended.
Exhibit M-2

