EX-10.7 11 d549494dex107.htm EX-10.7 CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE...
Exhibit 10.7
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
Execution Version
THIS LICENSE AGREEMENT (“Agreement”) is made effective as of the 3rd day of April, 2017 (the “Effective Date”), by and between Magenta Therapeutics, Inc., a Delaware corporation with its principal place of business located at 00 Xxxxxxxxx Xxxxxx, 0xx xxxxx, Xxxxxxxxx, XX 00000 (“LICENSEE”) and Novartis International Pharmaceutical Ltd., a for-profit corporation with its principal place of business at Xxxxxxxxxxxx 00, XX-0000 Xxxxx, Xxxxxxxxxxx (“NOVARTIS”). LICENSEE and NOVARTIS may, from time-to-time, be individually referred to as a “Party” and collectively referred to as the “Parties”.
RECITALS
WHEREAS, NOVARTIS Controls the Licensed Technology (hereinafter defined); and
WHEREAS, LICENSEE wishes to obtain, and NOVARTIS wishes to grant, certain licenses under the Licensed Technology on the terms and conditions set forth herein.
NOW, THEREFORE, in consideration of the mutual agreements and covenants set forth herein and other good and valuable consideration, the receipt and sufficiency of which the Parties hereby acknowledge, the Parties, intending to be legally bound hereby, agree to the foregoing and as follows:
| 1. | DEFINITIONS |
| 1.1 | “Accounting Standards” means, with respect to LICENSEE, United States Generally Accepted Accounting Principles, and, with respect to Novartis, International Financial Reporting Standards, in both cases as consistently applied throughout the Party’s organization. Each Party will promptly notify the other Party in the event that it changes the Accounting Standards pursuant to which its records relating to this Agreement are maintained; provided, however, that each Party may only use internationally recognized accounting principles (e.g., IFRS, US GAAP, etc.). |
| 1.2 | “Affiliate” means, with respect to a Party, any Person that controls, is controlled by, or is under common control with that Party. For the purpose of this definition, “control” shall refer to: (a) the possession, directly or indirectly, of the power to direct the management or policies of an entity, whether through the ownership of voting securities, by contract or otherwise; or (b) the ownership, directly or indirectly, of fifty percent (50%) or more of the voting securities of such entity. |
| 1.3 | “Applicable Laws” means all applicable laws, statutes, rules, regulations and guidelines, including all good manufacturing practices and all applicable standards or guidelines promulgated by the appropriate Regulatory Authority. |
| 1.4 | “Approval Application” means a BLA or similar application or submission for a Product filed with a Regulatory Authority in a country or group of countries to obtain marketing approval for a biological or pharmaceutical product in that country or group of countries. |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 1.5 | “Biologics License Application” or “BLA” means a Biologics License Application submitted to the FDA under subsection (a) of Section 351 of the PHSA or any corresponding foreign application in the Territory, including, with respect to the European Union, a marketing authorization application filed with the EMA pursuant to the centralized approval procedure or with the applicable Regulatory Authority of a country in Europe with respect to the mutual recognition or any other national approval. |
| 1.6 | “BLA-Enabling Clinical Trial” means (a) a Phase III Clinical Trial, or (b) a Clinical Trial regarding the efficacy and safety of a Product, which is designed to demonstrate statistically whether such Product is effective and safe for use in a particular Indication in a manner that generates data sufficient (by itself or together with other Clinical Trials) to permit the submission of a BLA for such Product, consistent with regional regulatory requirement for an accelerated approval pathway. To the extent that such approval is associated with conditional approval in Japan for a hematology oncology indication, which may be granted on the temporary basis with Phase 2 data, and that a further confirmatory Clinical Trial is required before the full approval can be granted in Japan, then 50% of the BLA-enabling Clinical Trial milestone will be due sixty (60) days after receipt of such conditional approval, with the other 50% becoming due within sixty (60) days after the date that the database for the confirmatory Clinical Trial has been locked. |
| 1.7 | “Business Day” means any day other than a Saturday, a Sunday or a day on which commercial banks located in Boston, Massachusetts are authorized or required by law to remain closed. |
| 1.8 | “Calendar Quarter” means the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 and December 31. |
| 1.9 | “Calendar Year” means any twelve (12) month period commencing on January 1. |
| 1.10 | “Change in Control” of a Person means (a) a merger, consolidation, reorganization, amalgamation, arrangement, share exchange, tender or exchange offer, private purchase, business combination, recapitalization or other transaction involving such Person that results in the stockholders of such Person immediately prior thereto ceasing to hold at least fifty percent (50%) of the outstanding shares, or less than fifty percent (50%) of the combined voting power of the surviving entity or the ultimate parent entity of the surviving entity immediately after such transaction, (b) a transaction or series of related transactions in which a Third Party, together with its Affiliates, becomes the beneficial owner of fifty percent (50%) or more of the combined voting power of the outstanding securities of such Person, (c) the sale or other transfer (in one transaction or a series of related transactions) to a Third Party of all or substantially all of such Person’s assets (or, in the case of a Party, all or substantially all of such Party’s business to which this Agreement relates), or (d) the adoption of a plan relating to the liquidation or dissolution of a Person, other than in connection with a corporate reorganization (without limitation of clause (a) above). |
2
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 1.11 | “Clinical Trials” means Phase I Clinical Trials, Phase II Clinical Trials, Phase III Clinical Trials or Phase IV Clinical Trials. |
| 1.12 | “Combination Product” means a therapeutic product that includes, incorporates, or embodies a Product together with at least one (1) Other Active Ingredient. |
| 1.13 | “Commercialize” or “Commercialization” means to manufacture for sale, market, promote, otherwise offer for sale, distribute or sell. |
| 1.14 | “Commercially Reasonable Efforts” means [***]. |
| 1.15 | “Compounds” means the compounds designated by NOVARTIS as [***] and [***], together with such other compounds as may be disclosed in the Licensed Patent Rights that claim [***] and [***]. |
| 1.16 | “Control” or “Controlled” means, with respect to any Intellectual Property Rights, the legal authority or right (whether by ownership, license or otherwise other than pursuant to this Agreement) of a Party to grant a license or a Sublicense of or under such Intellectual Property Rights to the other Party without breaching the terms of any agreement with a Third Party. |
| 1.17 | “Covered IP” means any Intellectual Property Rights that are related to and reasonably necessary for the Use of the Compounds or the Development, manufacture or Commercialization of any Product to the extent such activities are undertaken in connection with activities under this Agreement commencing with the manufacturing of such Compounds and ending with the packaging of cryopreserved Product (including all activities that would be undertaken between such commencing and ending activities). For the avoidance of doubt, Covered IP does not include Intellectual Property Rights solely for Use in conditioning regimens or transfusion protocols. |
| 1.18 | “Develop” or “Development” means all research, development and regulatory activities regarding a Product, including the Use of a Compound to manufacture a Product. Development shall include all preclinical and other nonclinical testing, test method development and stability testing, toxicology, formulation, process development, manufacturing scale-up, qualification and validation, quality assurance or quality control, Clinical Trials, manufacturing clinical supplies, regulatory affairs, statistical analysis, report writing, and all other activities necessary or reasonably useful or otherwise requested or required by a Regulatory Authority as a condition or in support of obtaining or maintaining Regulatory Approval. When used as a verb, “Develop” shall mean to engage in Development. |
3
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 1.19 | “Distributor” means a Third Party, other than a Third Party to which any Sublicense hereunder is granted, that (a) purchases any Products in finished form from LICENSEE or any of its Affiliates or Sublicensees with the intent or purpose of reselling such Products; and (b) has the right to Commercialize such Products in one or more regions. |
| 1.20 | “EMA” means the European Medicines Agency, or any successor agency thereto. |
| 1.21 | “European Markets” means the United Kingdom, France, Germany, Spain and Italy. |
| 1.22 | “FDA” means the United States Food and Drug Administration, or a successor federal agency thereto. |
| 1.23 | “FD&C Act” means the United States Federal Food, Drug, and Cosmetic Act, as amended, and the rules and regulations promulgated thereunder. |
| 1.24 | “Field” means all uses of HSCs other than Gene-Edited/-Modified HSCs. |
| 1.25 | “First Commercial Sale” means with respect to a Product, the first sale of such Product by LICENSEE or any of its Affiliates or Sublicensees to a Third Party for use or consumption of the Product following receipt of Regulatory Approval, for such Product in a country in the Territory. |
| 1.26 | “FPFD” means, with respect to a Clinical Trial, the first dosing of the first subject or patient in such Clinical Trial. |
| 1.27 | “GAAP” means the generally accepted accounting principles in the United States, consistently applied. |
| 1.28 | “Gene-Edited/-Modified HSC” means HSCs that have had their DNA or RNA altered by any means (other than naturally occurring mutations). |
| 1.29 | “Generic Equivalent” means, with respect to a Product in a country, any product that (a) has Regulatory Approval for use in such country pursuant to a regulatory process governing approval of generic, interchangeable or biosimilar pharmaceutical or biological product based on the then-current standards for regulatory approval in such country, where such Regulatory Approval relied on or incorporated clinical data generated by LICENSEE pursuant to this Agreement or was obtained using an abbreviated, expedited or other similar process; (b) during the Term, is not Controlled by LICENSEE (in the case of Products Commercialized by LICENSEE, its Affiliates, or their Sublicensees) under this Agreement, and (c) is sold in the same country as the relevant Product by a Third Party that is not a Sublicensee of LICENSEE (in the case of Products Commercialized by LICENSEE, its Affiliates, or their Sublicensees), and that did not purchase such product in a chain of distribution that included LICENSEE, or of any of its respective Affiliates or Sublicensees. |
4
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 1.30 | “Good Manufacturing Practices” means the then-current standards for good manufacturing practices for biological or pharmaceutical products (as applicable), as set forth in the FD&C Act and applicable regulations and guidance promulgated thereunder, including the Code of Federal Regulations, as amended from time to time, or under any other Applicable Laws. |
| 1.31 | “Governmental Authority” means any court, agency, department, authority or other instrumentality of any national, state, county, city or other political subdivision. |
| 1.32 | “Hemoglobinopathy Indication” means sickle cell disease or beta-thalassemia. |
| 1.33 | “HSC” means hematopoietic stem cells. |
| 1.34 | “IND” means: (a) an investigational new drug application filed with the FDA for authorization for the investigation of a Product; or (b) any foreign equivalents as filed with the applicable Regulatory Authorities in other countries or regulatory jurisdictions in the Territory, as applicable. |
| 1.35 | “Indication” for a Product means the use of such Product for treating a particular disease or medical condition. |
| 1.36 | “Information” means all technical, scientific and other know-how and information, trade secrets, knowledge, technology, means, methods, processes, practices, formulae, instructions, skills, techniques, procedures, experiences, ideas, technical assistance, designs, drawings, assembly procedures, computer programs, apparatuses, specifications, data, results and other material, including: biological, chemical, pharmacological, toxicological, pharmaceutical, physical and analytical, pre-clinical, clinical, safety, manufacturing and quality control data and information, including study designs and protocols, assays and biological methodology, in each case (whether or not confidential, proprietary, patented or patentable) in written, electronic or any other form now known or hereafter developed. |
| 1.37 | “Intellectual Property Rights” means all trade secrets, copyrights, Patents, Trademarks, moral rights, know-how and any and all other intellectual property or proprietary rights now known or hereafter recognized in any jurisdiction. |
| 1.38 | “Invention” means any invention, whether or not patentable, together with all intellectual property rights therein. |
| 1.39 | “Knowledge of Novartis” shall mean, with respect to NOVARTIS, [***]. |
| 1.40 | “Knowledge of the Novartis Deal Team” shall mean, with respect to NOVARTIS, the actual knowledge of [***]. |
| 1.41 | “Knowledge of the Patent Associates” shall mean, with respect to NOVARTIS, the actual knowledge of [***], in each case after review of such named individuals’ relevant files and records on the Effective Date. |
5
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 1.42 | “Licensed Know-How” means Information and regulatory filings, whether or not patentable, that are owned or Controlled by NOVARTIS or its Affiliates on the Effective Date and that are included in the Covered IP, which are described generally on Exhibit B-1 (as of the Effective Date or as amended pursuant to Section 3.2). |
| 1.43 | “Licensed Patent Rights” means all Patents that are owned or Controlled by NOVARTIS or its Affiliates on the Effective Date, and that are included in Covered IP for use in the Territory in the Field, including those Patents listed on Schedule A. |
| 1.44 | “Licensed Patent Rights Improvements” means Patents owned or Controlled by either Party or its Affiliates that are created, conceived of, or reduced to practice after the Effective Date that consist of improvements to the Licensed Technology; and that are Covered IP. |
| 1.45 | “Licensed Technology” means collectively, the Licensed Patent Rights and Licensed Know-How. |
| 1.46 | “Loss of Market Exclusivity” means, with respect to any Product in any country, (a) the Net Sales of such Product in that country in any Calendar Quarter are at least [***] less, as compared with the Net Sales of such Product in that country in the Calendar Quarter immediately preceding the marketing or sale of the first Generic Equivalent of such Product; and (b) the decline in sales is reasonably attributable in material part to the availability of a Generic Equivalent in such market. |
| 1.47 | “MAA” means (a) a Marketing Authorization Application for a Product filed with (i) the EMA under the centralized European procedure (including amendments and supplements thereto) or (ii) a Regulatory Authority in any country in the European Union if the centralized European procedure is not used to obtain Regulatory Approval of such Product; or (b) any other equivalent or related Regulatory Filing, such as a Type II variation, to gain Regulatory Approval of a Product in any country in the European Union. |
| 1.48 | “Major Markets” means the United States, the United Kingdom, France, Germany, Spain, Italy and Japan. |
| 1.49 | “Milestone” means each milestone set forth in Section 5.2. |
| 1.50 | “Net Sales” means the net sales recorded by LICENSEE or any of its Affiliates or Sublicensees for any Product sold to Third Parties other than Sublicensees, as determined by computing the gross sales of such Product and deducting the following amounts, in all cases to the extent permitted by LICENSEE’s Accounting Standards, as consistently applied, [***]. |
| 1.51 | “Other Active Ingredient” means any therapeutically active pharmaceutical ingredient other than a Product. |
6
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 1.52 | “Other Indication” means an Indication that is not an Ultra Orphan Indication or an Hemoglobinopathy Indication. |
| 1.53 | “Patents” means: (a) all national, regional and international patents and patent applications, including provisional patent applications; (b) all patent applications filed either from such patents, patent applications or provisional applications or from an application claiming priority from either of these, including divisionals, continuations, continuations-in-part, provisionals, converted provisionals and continued prosecution applications; (c) any and all patents that have issued or in the future issue from the foregoing patent applications ((a) and (b)), including utility models, xxxxx patents, innovation patents and design patents and certificates of invention; (d) any and all extensions or restorations by existing or future extension or restoration mechanisms, including revalidations, reissues, re-examinations and extensions (including any supplementary protection certificates and the like) of the foregoing patents or patent applications ((a), (b) and (c)); and (e) any similar rights, including so-called pipeline protection or any importation, revalidation, confirmation or introduction patent or registration patent or patent of additions to any of such foregoing patent applications and patents. |
| 1.54 | “Person” means an individual, corporation, partnership, limited liability company, trust, business trust, association, joint stock company, joint venture, pool, syndicate, sole proprietorship, unincorporated organization, Governmental Authority or any other form of entity not specifically listed herein. |
| 1.55 | “Phase I Clinical Trial” means a human clinical trial of a product, the principal purpose of which is a determination of initial tolerance or safety of such product in healthy volunteers and/or the target patient population, as described in 21 CFR 312.21(a) (as amended or any replacement thereof), or a similar clinical trial prescribed by the Regulatory Authority in a country other than the United States. |
| 1.56 | “Phase II Clinical Trial” means a human clinical trial of a product, the principal purpose of which is a determination of safety and efficacy in the target patient population, as described in 21 C.F.R. 312.21(b) (as amended or any replacement thereof), or a similar clinical trial prescribed by the Regulatory Authority in a country other than the United States. |
| 1.57 | “Phase III Clinical Trial” means a human clinical trial of a product, the design of which is acknowledged by the FDA to be sufficient for such clinical trial to satisfy the requirements of 21 C.F.R. 312.21(c) (as amended or any replacement thereof), or a similar human clinical trial prescribed by the Regulatory Authority in a country other than the United States, the design of which is acknowledged by such Regulatory Authority to be sufficient for such clinical trial to satisfy the requirements of a pivotal efficacy and safety clinical trial. |
| 1.58 | “Phase IV Clinical Trial” means any study of a product following the first Regulatory Approval for the sale of such product whether or not required by a Governmental Authority. Phase IV Clinical Trials may include epidemiological studies, modeling and pharmacoeconomic studies, postmarketing surveillance studies and clinical or other research studies. |
7
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 1.59 | “Product” means cord blood derived non-Gene-Edited/-Modified HSCs, expanded in the presence of the Compounds, or created through the use of Licensed Know-How, in any and all dosage forms, presentations and formulations. |
| 1.60 | “Progress Milestone” means [***]. |
| 1.61 | “Regulatory Approval” means, with respect to a Product in any country or regulatory jurisdiction, any approval (including where required or otherwise reasonably necessary to determine commercial viability, pricing and reimbursement approvals), registration, license or authorization that is required by the applicable Regulatory Authority to market and sell such Product in such country or regulatory jurisdiction. |
| 1.62 | “Regulatory Authority” means any Governmental Authority responsible for granting Regulatory Approvals for a Product in the Territory. |
| 1.63 | “Regulatory Filings” means, with respect to a Product, any submission to a Regulatory Authority of any appropriate regulatory application, including, without limitation, any IND, any BLA, any submission to a regulatory advisory board, any marketing authorization application (including any MAA), and any supplement or amendment thereto. |
| 1.64 | “Royalty Term” means, on a Product-by-Product and country-by country basis, the period commencing on the First Commercial Sale of such Product in such country and expiring upon the later of: (a) expiration of the last Valid Claim of a Licensed Patent Right that covers the manufacture, use, or sale of such Product or a Compound used in the manufacture of such Product in such country; or (b) ten (10) years following the date of First Commercial Sale of such Product in such country. |
| 1.65 | “Sublicense” means a bona fide, arms length agreement (other than a permitted assignment of this Agreement) in which a Party (a) grants or otherwise transfers any of the rights licensed to such Party hereunder, (b) agrees not to assert such rights or to xxx, prevent or seek a legal remedy for the performance or practice of same, or (c) is under an obligation to grant, assign or otherwise transfer any such rights or non-assertion, or to forebear from granting or otherwise transferring such rights to any other entity. Agreements expressly considered Sublicenses include (x) licenses, option agreements, “lock up” agreements, right of first refusal agreements, non-assertion agreements, covenants not to xxx, distribution agreements that grant or otherwise transfer any rights licensed to a Party hereunder, or similar agreements and (y) agreements that grant or otherwise transfer rights licensed to a Party under this Agreement along with rights owned by such Party or granted to such party by a Third Party. For the avoidance of doubt, if a Sublicense is entered into pursuant to an option or similar agreement that is also a Sublicense, then the date of the exercise of the option or similar agreement shall be deemed the execution date, not the date of the execution of the Sublicense. |
8
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 1.66 | “Sublicense Income” shall mean consideration in any form that LICENSEE or any of its Affiliates receives in consideration of a Sublicense of any Product under such Sublicense. Sublicense Income shall include any license fee, license maintenance fee, option fee, milestones (other than sales milestones relating to Net Sales of greater than [***]) and fair market value of equity securities received by LICENSEE or an Affiliate in consideration of such Sublicense that is not issued in exchange for any cash payment; provided that in the event a milestone payment is due to LICENSEE under a Sublicense, on the one hand, and to NOVARTIS under this Agreement, on the other hand, for the same or reasonably similar milestone event, the amount of Sublicense Income that LICENSEE receives shall be deemed to be the difference between the milestone payment payable under the Sublicense minus the Milestone Payment due under this Agreement to NOVARTIS. In the event LICENSEE or an Affiliate receives non-monetary consideration in connection with a Sublicense, Sublicense Income shall be calculated based on the fair market value of such consideration at the time of the transaction assuming an arm’s length transaction made in the ordinary course of business. |
| Sublicense Income specifically excludes the following: |
[***]
| 1.67 | “Sublicensee” shall mean any entity to which LICENSEE or its Affiliate has granted a Sublicense. |
| 1.68 | “Territory” means worldwide. |
| 1.69 | “Third Party” means any Person other than a Party or an Affiliate of a Party. |
| 1.70 | “TSRI” means The Scripps Research Institute. |
| 1.71 | “TSRI Agreement” means the license agreement by and between TSRI and Novartis Pharmaceuticals Corporation, dated May 30, 2008, pursuant to which certain of the Licensed Patent Rights and Licensed Know-How were licensed to Novartis’ Affiliate. |
| 1.72 | “TSRI IP” means the portion of the Licensed Patent Rights and Licensed Know-How that were licensed to Novartis’ Affiliate pursuant to the TSRI Agreement. |
| 1.73 | “Trademarks” means all registered and unregistered trademarks, service marks, trade dress, trade names, logos, insignias, domain names, symbols, designs, and combinations thereof. |
9
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 1.74 | “Ultra Orphan Indication” means (i) the diseases or medical conditions included in the three families of diseases or medical conditions set forth on Schedule 1.74, to the
extent the various diseases or medical conditions are expressly identified on that schedule; and (ii) any other Indication for a disease or medical condition with a prevalence of ten (10) cases or less per one hundred thousand (100,000)
persons as published by either one (or both) of Orphanet or the National Institutes of Health National Library of Medicine. For purposes of this Section 1.74, any group of Indications for which a single Regulatory Approval may be obtained shall
be considered a single Indication, and if each Indication in such group of Indications meets the criteria described in clause (ii) above, such group of Indications shall together be considered a single Ultra Orphan Indication, unless the total
prevalence of all diseases in the group exceeds 30 in 100,000. If the patient numbers in a relevant publication are given in a range, then for the purpose of determining the prevalence of the disease, the geometric mean of such range will be used
(i.e., the following formula will be used:
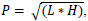 where P is the total prevelance for the purpose of this definition, L is the low end (minimum) of the relevant prevelance range, and H is the high end (maximum) of the relevant prevelance range).
where P is the total prevelance for the purpose of this definition, L is the low end (minimum) of the relevant prevelance range, and H is the high end (maximum) of the relevant prevelance range).
|
| 1.75 | “Use” means to research, develop, make, have made, use, sell, offer for sale, market, distribute, import, export or otherwise exploit. |
| 1.76 | “Valid Claim” means either: (a) a claim of an issued and unexpired Patent included within the Licensed Patent Rights, which has not been permanently revoked or declared unenforceable or invalid by an unreversed and unappealable or unreversed and unappealed decision of a court or other appropriate body of competent jurisdiction and which has not been abandoned, disclaimed, denied or admitted to be invalid or unenforceable through reissue, re-examination, disclaimer or otherwise; or (b) a claim of a pending patent application included within the Licensed Patent Rights that has been pending for less than seven (7) years from the earliest claimed priority date, which claim was filed in good faith and has not been cancelled, withdrawn, abandoned or finally disallowed without the possibility of appeal or refiling of such application. |
| 1.77 | Additional Definitions. Each of the following definitions is set forth in the Section indicated below: |
| Definition | Section | |
| AAA | 16.2 | |
| Agreement | Preamble | |
| Allocation Arbitrator | 5.2.6(c) | |
| Allocation Dispute | 5.2.6(b) | |
| Allocation Notice | 5.2.6(b) | |
| Allocation Notice Period | 5.2.6(b) | |
| Arbitrator | 16.2 | |
| Bankruptcy Code | 13.4 |
10
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| Bankruptcy Event | 13.4 | |
| CDA | 17.11 | |
| Certificate of Analysis | 4.4.1(b) | |
| Claims | 11.1 | |
| Defense Action | 8.1 | |
| Designated Affiliate/Third Party | 13.6.4(e) | |
| Developed IP | 7.2 | |
| Effective Date | Preamble | |
| Existing Supply | 4.4.1(a) | |
| Fees | 6.1.1 | |
| Force Majeure Event | 17.4 | |
| HemOnc IND | 4.3.1(a) | |
| IMD IND | 4.3.1(b) | |
| Indemnification Claim Notice | 11.3.1 | |
| Indemnified Party | 11.3.1 | |
| Indemnifying Party | 11.3.1 | |
| LICENSEE | Preamble | |
| LICENSEE Indemnitees | 11.2 | |
| LICENSEE Inventory | 13.6.4(e) | |
| Losses | 11.1 | |
| Milestone | 5.2.1 | |
| Milestone Payment | 5.2.1 | |
| Multi-Product Sublicense | 5.2.6(b) | |
| Notice Period | 13.2.1 | |
| NOVARTIS | Preamble | |
| NOVARTIS Indemnitees | 11.1 | |
| Part(ies) | Preamble | |
| Pharmacovigilance Agreement | 4.3.3 | |
| Proposed Allocation | 5.2.6(b) | |
| Proposed Dispute Allocation | 5.2.6(c) | |
| Public Company | 5.1.2 | |
| Recipients | 9.2 | |
| Relevant Records | 6.1.1 | |
| Remaining Recoveries | 8.2.4 | |
| Reversion IP | 13.6.4(a) | |
| Royalty Payment | 5.2.5(a)(vi) | |
| Series A Preferred Stock | 5.1.1 | |
| Series B Financing | 5.1.2 | |
| Series B Preferred Stock | 5.1.2 | |
| Specifications | 4.4.1(b) | |
| Subsequent Shares | 5.1.2 | |
| Term | 13.1 | |
| Third Party Infringement | 8.1 | |
| Third Party IP | 5.2.5(b) | |
| Third Party Payment | 5.2.5(b) | |
| Transfer Activities | 3.1 | |
| Upfront Shares | 5.1.1 | |
| VAT | 5.4.1 |
11
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 2. | LICENSES AND IMPROVEMENTS. |
| 2.1 | License Grant. |
| 2.1.1 | Licensed Technology. NOVARTIS hereby grants to LICENSEE a sublicensable (subject to Section 2.2), royalty-bearing right and license under the Licensed Technology and under all Information contained in the HemOnc IND (a) to research, Develop, and manufacture Compounds for the purpose of Using such Compounds to manufacture and Commercialize Products in the Field in the Territory, and (b) to research, Develop, manufacture, and Commercialize Products in the Field in the Territory, including, for the avoidance of doubt, in the case of Information contained in the HemOnc IND, to include such Information in Regulatory Filings filed by LICENSEE, its Affiliates or Sublicensees in connection with the research, Development or manufacture of Compounds or Products in the Field in the Territory. The license granted under this Section 2.1.1 shall be exclusive even as to NOVARTIS and its Affiliates with respect to Products in the Field; provided, however, that (a) TSRI retains the right to practice and Use the TRSI IP for its own research and educational purposes, and retains the right to grant licenses to the TSRI IP to other non-profit organizations, government agencies, and universities for their research and educational purposes; (b) NOVARTIS and its Affiliates will retain the right to practice and Use the Licensed Technology in the Field for internal research (but not Development or Commercialization) purposes; and (c) LICENSEE acknowledges that portions of the TSRI IP may be considered “Subject Inventions” under 35 U.S.C. § 200-212, and as a result, the United States Government retains certain rights to the TSRI IP under Applicable Law. For the avoidance of doubt, Novartis and its Affiliates will retain the right to Use and practice the Licensed Technology other than for the purpose of manufacturing, Developing, and Commercializing Products in the Field. |
| 2.1.2 | Affiliates. To the extent that any of the Licensed Technology is Controlled by an Affiliate of NOVARTIS (including but not limited to, e.g., the TSRI IP), then promptly following the Effective Date, NOVARTIS shall procure that such Affiliate undertakes all necessary actions to give effect to the licenses granted under this Section 2.1. |
| 2.2 | Cross License to Licensed Patent Rights Improvements. |
| 2.2.1 | Upon LICENSEE’s written request, which must identify with specificity the relevant Patent Right, NOVARTIS hereby grants to LICENSEE a fully paid and royalty-free, non-exclusive license to practice any Licensed Patent Rights Improvements Controlled by NOVARTIS (a) to research, |
12
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| Develop, and manufacture Compounds for the purpose of Using such Compounds to manufacture and Commercialize Products in the Field in the Territory, and (b) to research, Develop, manufacture, and Commercialize Products in the Field in the Territory. |
| 2.2.2 | Upon NOVARTIS’s written request, which must identify with specificity the relevant Patent Right, LICENSEE hereby grants to NOVARTIS and its Affiliates a fully paid and royalty-free, non-exclusive license to practice any Licensed Patent Rights Improvements Controlled by LICENSEE (a) to research, Develop, and manufacture Compounds for the purpose of Using such Compounds to manufacture and Commercialize HSC products outside the Field in the Territory, and (b) to research, Develop, manufacture, and Commercialize HSC products made Using such Compounds outside the Field in the Territory. |
| 2.2.3 | For clarity, nothing in this Section 2.2 grants to either Party any license to Know-How arising after the Effective Date, and neither Party will have any obligation to inform the other Party of the existence of any Licensed Patent Rights Improvements or to conduct any technology transfer or to provide any assistance with respect to any Licensed Patent Rights Improvements. |
| 2.3 | Sublicense Rights. Each Party shall have the right to Sublicense the rights granted to it by the other Party under this Agreement (including rights under Section 2.1 and Section 2.2), through multiple tiers of Sublicensees to its Affiliates or any Third Party, provided that (a) any such Sublicenses shall be consistent with the terms and conditions of this Agreement, (b) each Party shall be responsible for the acts and omissions of its Sublicensees as if such Sublicensees were the relevant Party hereunder, and (c) if a Party licenses or Sublicenses (as applicable) all or substantially all of its rights under this Agreement or, in the case of NOVARTIS, its retained rights in the Licensed Technology (e.g., if LICENSEE Subicenses all of its rights to Develop and Commercialize all HSCs in the Field or NOVARTIS licenses all of its remaining rights in the Licensed Technology), then, as a condition to such Sublicense or license, the Sublicensee or licensee must agree to be bound by the provisions of Section 2.2.1 or Section 2.2.2 (as applicable) as if such licensee or Sublicensee were NOVARTIS or LICENSEE (as applicable). A Party granting a Sublicense pursuant to this Section 2.3 shall furnish to the other Party a true and complete copy of each Sublicense and each amendment thereto, within thirty (30) days after the Sublicense or amendment has been executed, which copy may be redacted by the Party granting the Sublicense to remove proprietary and confidential information to the extent not required to confirm compliance with this Section 2.3; provided that neither Party shall be required to provide copies of any Sublicenses with Third Parties that are solely performing services on behalf of such Party (which, for the avoidance of doubt, shall not include Sublicenses with academic investigators and collaborators). The terms of any Sublicense disclosed to the other Party pursuant to this Section 2.3 shall be deemed the Confidential Information of the relevant Party granting the Sublicense. |
13
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 3. | TRANSFER ACTIVITIES |
| 3.1 | Technology Transfer and Transition Services. NOVARTIS shall (a) transfer to LICENSEE the embodiments of the Licensed Technology set forth in Schedule B-2; and (b) perform the services set forth in Schedule B-2 (where the activities under subsections (a) and (b) shall be collectively referred to as “Transfer Activities”). NOVARTIS shall use commercially reasonable efforts to perform the Transfer Activities and complete such Transfer Activities within the time periods specified in Schedule B-2. |
| 3.2 | Subsequently Identified Licensed Technology. To the extent that LICENSEE identifies in writing to Novartis, within six (6) months after the Effective Date, Licensed Know-How that was not identified in Schedule B-1 as of the Effective Date, the Parties will update Schedule B-1 to add such Licensed Know-How, to the extent such proposed Licensed Know-How (a) was owned or Controlled by NOVARTIS or its Affiliates as of the Effective Date; and (b) relates specifically to the Licensed Patent Rights or Licensed Know-How as of the Effective Date. Notwithstanding the foregoing, in no event will Schedule B-1 be amended to add (and Novartis will not be required to conduct Transfer Activities with respect to) (i) HSC formulations other than commercially available liquid or cryopreserved formulations; (ii) manufacturing methods other than “Process B Cryopreserved”; (iii) conditioning regimens; (iv) transfusion protocols; (v) methods of manufacturing Products (other than those that have been previously transferred to or developed at the Molecular and Cellular Therapies group at the University of Minnesota); or (vi) except where expressly provided in Schedule B-1, any reports other than “finalized” or “final” reports. |
| 4. | DEVELOPMENT, MANUFACTURING, REGULATORY AND COMMERCIALIZATION |
| 4.1 | Development. |
| 4.1.1 | LICENSEE shall itself, or through its Affiliates or Sublicensees, use Commercially Reasonable Efforts to Develop Products in the Major Markets in the Field, and LICENSEE shall undertake all Development activities relating to the Compounds and Products in the Field at its sole expense. Without limiting the foregoing, in connection with its efforts to Develop Products, LICENSEE shall bear all responsibility and expense for filing Regulatory Filings in LICENSEE’s name and obtaining Regulatory Approval for Products. Until LICENSEE obtains Regulatory Approval for a Product in a country in the Territory, LICENSEE shall provide to NOVARTIS reports summarizing LICENSEE’s Development results annually during the term of this Agreement (including progress and development) and LICENSEE will make appropriate personnel available |
14
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| telephonically once per year to discuss such reports with NOVARTIS. LICENSEE shall provide each such report to NOVARTIS no later than December 31st of each year until such reports are no longer required pursuant to this Section 4.1.1. Novartis will not consent for TSRI to conduct any Clinical Trials in the Field pursuant to Section 4.3 of the TSRI Agreement. |
| 4.2 | Commercialization. LICENSEE shall itself, or through its Affiliates, Sublicensees or Distributors, use Commercially Reasonable Efforts to Commercialize the Products in those Major Market countries where it has received Regulatory Approval, it being understood that LICENSEE, in the exercise of such Commercially Reasonable Efforts, may determine to not Commercialize the Product in certain Major Market countries. LICENSEE shall undertake such activities at its sole expense and shall have sole decision-making authority with respect to such activities. |
| 4.3 | Regulatory and Pharmacovigilance. |
| 4.3.1 | Right of Reference of Regulatory Filings; Assignment of IND. |
| (a) | NOVARTIS hereby grants to LICENSEE and any Affiliate, Sublicensee or other designee of LICENSEE the right to reference the HemOnc IND (#14822) (the “HemOnc IND”) for the purpose of Developing, and seeking, obtaining, and maintaining Regulatory Approval for, manufacturing and otherwise exploiting Products in the Field in the Territory. Novartis will maintain the HemOnc IND as “open” until March 1, 2019. NOVARTIS will provide a copy of the HemOnc IND in accordance with the provisions set forth on Schedule B-2. NOVARTIS shall, promptly following any request by LICENSEE, provide notifications reasonably requested by LICENSEE to Regulatory Authorities identified by LICENSEE. If, after March 1, 2019, NOVARTIS intends to close the HemOnc IND, it will give ninety (90) days’ written notice of such intention to LICENSEE, and if LICENSEE requests that the HemOnc IND be transferred to it during that time period, NOVARTIS shall assign to LICENSEE the HemOnc IND and all associated Regulatory Filings in a manner consistent with the provisions set forth for the assignment of the IMD IND (described in Schedule B-2). |
| (b) | NOVARTIS shall assign to LICENSEE the Inherited Metabolic Disease IND (#16729) (the “IMD IND”) and all associated United States Regulatory Filings. NOVARTIS will transfer such Regulatory Filings, documents, records, and data regarding the IMD IND in accordance with the provisions set forth on Schedule B-2. |
15
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| (c) | LICENSEE shall use commercially reasonable efforts to seek Regulatory Approval for Products in the Major Markets, it being understood that LICENSEE, in the exercise of such Commercially Reasonable Efforts, may determine to not seek Regulatory Approval for Product in certain Major Market countries. |
| 4.3.2 | Safety Report. NOVARTIS shall submit NOVARTIS-generated safety reports for all Compounds to the relevant Regulatory Authorities until the effective date of the transfer of the IMD IND to LICENSEE. |
| 4.3.3 | Pharmacovigilance and Regulatory Reporting Agreement. The Parties shall cooperate with regard to the reporting and handling of safety information involving or relating to the Compounds and/or the Products to the extent required by Applicable Laws. Following the Effective Date, in time to ensure that all regulatory requirements are met, and to the extent required by Applicable Laws or any Regulatory Authority, the Parties will enter into a written agreement that will govern the exchange of adverse event and other safety information and the Parties’, their Affiliates’ and their Sublicensees’ respective reporting obligations relating to the Compounds and/or the Products (the “Pharmacovigilance Agreement”). Such Pharmacovigilance Agreement shall ensure that adverse events and other safety information is exchanged and reported to the relevant Regulatory Authorities upon terms that will permit each Party to comply with Applicable Laws and requirements of Regulatory Authorities. |
| 4.3.4 | Regulatory Cooperation. If a Regulatory Authority contacts NOVARTIS regarding an audit of any of the research and development done prior to the Effective Date, by, or under the direction of, NOVARTIS regarding HSC835, NOVARTIS shall, without undue delay, notify LICENSEE and shall respond to the query or allow the Regulatory Authority to audit the relevant data as may be requested; provided that NOVARTIS will provide LICENSEE with a reasonable amount of time to provide comments regarding any such response and shall consider in good faith all such comments. If a Regulatory Authority contacts LICENSEE regarding an audit of any of the research and development done prior to the Effective Date, by, or under the direction of, NOVARTIS regarding HSC835, NOVARTIS shall use reasonable efforts to provide reasonable assistance to LICENSEE to respond to the query or allow the Regulatory Authority to audit the relevant data as may be requested. As long as Novartis retains all source documents in the HemOnc IND, NOVARTIS will provide reasonable assistance to assist LICENSEE to prepare responses to any Regulatory Agency questions related to NOVARTIS-generated data. Such services will be billed to LICENSEE at the rate of [***]. |
16
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 4.4 | Manufacturing. LICENSEE shall have the sole right to manufacture, or have manufactured, Products in the Field, and it shall be entitled to use, and to Sublicense the manufacturing rights under the Licensed Patent Rights, for such purposes. Except as provided below, LICENSEE shall be responsible for all aspects of manufacturing of the Compounds and Products. LICENSEE acknowledges that a portion of the TSRI IP may be considered “Subject Inventions” under 35 U.S.C. § 200-212, and as a result, Applicable Law may require LICENSEE to manufacture Products covered by the TRSI IP in the United States, unless a waiver of such obligation is obtained by LICENSEE. |
| 4.4.1 | Transfer of Inventory. |
| (a) | At LICENSEE’s request, which request must be made no later than 60 days after the Effective Date and for a period of not to exceed 30 days from the first date of any such request, NOVARTIS will make available to LICENSEE nine hundred (900) vials of LDH221 (2.2mg/ml liquid, in vial 1ml (DS batch 101003005, DP batch Y0920614)), to the extent in Novartis’s or its Affiliates possession and in the form in Novartis’ or its Affiliates’ possession as of the date of LICENSEE’s request (the “Existing Supply”). The Existing Supply will be made available Ex Works (NOVARTIS’ facility in Basel, Switzerland) (Incoterms 2010) for no more than two shipments, and LICENSEE shall assume all responsibility for shipping, insuring, and receiving the Existing Supply from that facility. The Existing Supply will not be used for the Commercialization of a Product. LICENSEE (a) will use such Existing Supply solely for performance of the research and Development of Products, under suitable containment conditions in accordance with all Applicable Laws, as well as with all guidelines for use of the Existing Supply provided by NOVARTIS; (b) will under no circumstances administer the Existing Supply to humans (except as may be incidentally included in a Product); and (c) will use the Existing Supply with caution and prudence in any experimental work, since not all of the characteristics of such Existing Supply are necessarily known. Subject to NOVARTIS’ compliance with Section 4.4.1(b), LICENSEE shall bear all risk to it and/or any others resulting, directly or indirectly, from shipping, receipt, use, application, storage, disposal, and destruction of the Existing Supply. |
| (b) | At the time of transfer of any Existing Supply to LICENSEE, each shipment of Existing Supply (i) will have been manufactured in accordance with all Applicable Laws in effect at the time of manufacture, (ii) will have been manufactured under Good Manufacturing Practices; (iii) shall conform to specifications to be provided to LICENSEE upon shipment (the “Specifications”). For the avoidance of doubt, the Specifications shall only define the Existing Supply in the form specified above, and no warranty is provided that the Specifications will be fit for LICENSEE’s |
17
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| purposes and no warranty is given with respect to any other forms, strengths, or formulations. NOVARTIS shall provide to LICENSEE a certificate of analysis (a “Certificate of Analysis”) with each shipment of Existing Supply stating that such Existing Supply conforms to such Specifications. Such Certificate of Analysis shall include (i) the manufacturing date and the expiration date for the Existing Supply and (ii) a statement confirming that such Existing Supply was manufactured in accordance with Good Manufacturing Practices. |
| 5. | PAYMENT TERMS |
| 5.1 | Equity. |
| 5.1.1 | In partial consideration of the licenses and rights granted to LICENSEE hereunder, LICENSEE shall issue to NOVARTIS on the Effective Date a number of shares of LICENSEE’s Series A Preferred Stock (the “Series A Preferred Stock”) equal to $2,500,000 divided by the original issue price per share of the Series A Preferred Stock (the “Upfront Shares”). In connection with the issuance of the Upfront Shares, NOVARTIS shall become a party to all other agreements to which other holders of the Series A Preferred Stock have become parties in connection with their purchase of Series A Preferred Stock together with such other customary documents as LICENSEE may reasonably request, [***]. |
| 5.1.2 | In partial consideration of the licenses and rights granted to LICENSEE hereunder, LICENSEE shall, upon the earlier of (i) the closing of a transaction by LICENSEE (the “Series B Financing”) whereby LICENSEE issues a new series of Preferred Stock of Licensee (the “Series B Preferred Stock”) or (ii) December 31, 2017, issue additional shares of its capital stock accordance with this Section 5.1.2. In the event of a Series B Financing, LICENSEE shall issue to NOVARTIS a number of shares of Series B Preferred Stock equal to $2,500,000 divided by the original issue price of the Series B Preferred Stock no later than thirty (30) days after the closing of the Series B Financing. In the event that a Series B Financing has not occurred prior to December 31, 2017, LICENSEE shall issue to NOVARTIS, no later than January 15, 2018, the number of shares of Series A Preferred Stock equal to the number of Upfront Shares issued pursuant to Section 5.1.1 (in either case, such shares are referred to as “Subsequent Shares”); provided, however, [***]. Notwithstanding the foregoing, if LICENSEE has consummated an initial public offering of its common stock or otherwise has a class of capital stock registered under the Securities Exchange Act of 1934, as amended (the foregoing hereinafter referred to as being a “Public Company”) prior to December 31, 2017, or if LICENSEE has undergone a Change of Control prior to December 31, 2017, then, in lieu of the issuance of the Subsequent Shares, LICENSEE shall pay to NOVARTIS, not later than thirty (30) days after becoming a Public Company or completing a Change of Control (as applicable) $2,500,000. |
18
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 5.2 | Milestones and Royalty Payments. |
| 5.2.1 | Milestone Payments. LICENSEE shall notify NOVARTIS within [***] days after achievement of each Milestone described in Sections 5.2.2, 5.2.3 and 5.2.4 (each, a “Milestone”). In further consideration of the licenses and rights granted to LICENSEE, within [***] days after achievement of each Milestone set forth below (unless otherwise specified below), LICENSEE shall, subject to Section 1.6, pay to NOVARTIS the corresponding non-creditable and non-refundable milestone payment (each, a “Milestone Payment”). For the avoidance of doubt each Milestone Payment shall be payable only once upon achievement of the applicable Milestone. |
| 5.2.2 | Development Milestones. |
| DEVELOPMENT MILESTONE | MILESTONE PAYMENT | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] |
| (a) | Notwithstanding the foregoing, on a Milestone by Milestone basis, instead of paying the Milestone Payment for any Development Milestone set forth in this Section 5.2.2 in cash, LICENSEE may elect, at its sole discretion, to issue to NOVARTIS shares of the series of preferred stock then most recently issued by LICENSEE, if such Milestone is achieved and payment is due prior to LICENSEE becoming a Public Company and prior to a Change of Control. The number of shares to be issued pursuant to this clause shall be equal to the amount of the Milestone owed to NOVARTIS divided by the issuance price of the then-most recent series of preferred stock of LICENSEE. In connection with such issuance, NOVARTIS shall enter into any agreement and execute any customary document that LICENSEE may reasonably request provided, however, [***]. |
19
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 5.2.3 | Regulatory Milestones. |
| REGULATORY MILESTONES | MILESTONE PAYMENT | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] |
20
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 5.2.4 | Sales Milestones. |
| SALES MILESTONES | MILESTONE PAYMENT | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] | |
| [***] | [***] |
| (a) | [***]. The development Milestones provided for in this Section 5.2.4 shall be due and payable within [***] days after the end of the Calendar Quarter in which the relevant Sales Milestone has been met. |
| 5.2.5 | Royalty Payments. |
| (a) | In consideration of the licenses and rights granted to LICENSEE hereunder, LICENSEE shall pay to NOVARTIS, with respect to sales of the Products in the Territory during the applicable Royalty Term, an amount equal to: |
| (i) | [***] of Net Sales in a Calendar Year (or portion thereof) for the portion of annual aggregate Net Sales of the Products in the Territory (aggregated in all countries with respect to which the Royalty Term for such Products has not expired) below or equal to [***]; plus |
| (ii) | [***] of Net Sales in a Calendar Year (or portion thereof) for the portion of annual aggregate Net Sales of the Products in the Territory (aggregated in all countries with respect to which the Royalty Term for such Products has not expired) greater than [***] and less than or equal to [***]; plus |
| (iii) | [***] of Net Sales in a Calendar Year (or portion thereof) for the portion of annual aggregate Net Sales of the Products in the Territory (aggregated in all countries with respect to which the Royalty Term for such Products has not expired) greater than [***] and less than or equal to [***]; plus |
21
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| (iv) | [***] of Net Sales in a Calendar Year (or portion thereof) for the portion of annual aggregate Net Sales of the Products in the Territory (aggregated in all countries with respect to which the Royalty Term for such Products has not expired) greater than [***] and less than or equal to [***]); plus |
| (v) | [***] of Net Sales in a Calendar Year (or portion thereof) for the portion of annual aggregate Net Sales of the Products in the Territory (aggregated in all countries with respect to which the Royalty Term for such Products has not expired) greater than [***] and less than or equal to [***]; plus |
| (vi) | [***] of Net Sales in a Calendar Year (or portion thereof) for the portion of annual aggregate Net Sales of the Products in the Territory (aggregated in all countries with respect to which the Royalty Term for such Products has not expired) in excess of [***] (each, a “Royalty Payment”). |
| LICENSEE shall pay such Royalty Payments to NOVARTIS within [***] days following the end of each Calendar Quarter after the date of the First Commercial Sale. |
| (b) | If, during the Term, LICENSEE determines in good faith that it is reasonably necessary to obtain any Third Party’s Intellectual Property Rights (“Third Party IP”) in order to Develop, Commercialize or manufacture a Compound or Product, then in the event LICENSEE or any of its Affiliates or Sublicensees obtains a license under or acquires such Third Party IP, and if LICENSEE or any of its Affiliates or Sublicensees pays any amounts to such Third Party in connection with a license under or the acquisition of such Third Party IP, including upfront payments, milestones or royalties (the “Third Party Payment”), then LICENSEE may credit [***] of such Third Party Payment made in a given Calendar Quarter against the Royalty Payments owed and payable on the Net Sales for the Product for such Calendar Quarter. Notwithstanding the foregoing, in no event shall such credits reduce the royalties payable to NOVARTIS to less than [***] of the Royalty Payments that would otherwise be owed on such Net Sales prior to the application of such credits; provided that any permitted credits under this Section 5.2.5(b) that are not fully used by LICENSEE against any royalties payable under Section 5.2.5 in |
22
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| a particular Calendar Year to reduce the Royalty Payments in such Calendar Year may be carried over to Royalty Payments in subsequent Calendar Years, until fully used in accordance with this Section 5.2.5(b). Notwithstanding the foregoing, if the TSRI Agreement is terminated, and if LICENSEE obtains a direct license to the TSRI Technology (as defined in the TSRI Agreement) following such termination, LICENSEE may deduct any payment paid to TSRI under such agreement from any payment due to Novartis under this Agreement. |
| (c) | If a Loss of Market Exclusivity for any Product occurs in any country in the Territory, and for so long as the Loss of Market Exclusivity continues, the Net Sales of such Product for such country, for the purpose of the calculation of Royalty Payments due under Section 5.2.5 will be reduced by [***]. |
| (d) | During the Royalty Term, the operation of Sections 5.2.5(b) and 5.2.5(c) individually or in combination, shall not reduce by more than [***] the Royalty Payments that would otherwise have been owed under Section 5.2.5 for a Product in the Territory in any Calendar Quarter. |
| (e) | Commencing on the First Commercial Sale of a Product, LICENSEE will provide reports on a Calendar Quarter basis (with each such report to be delivered within [***] days after the end of such Calendar Quarter), which will include a total quarterly sales calculation of gross sales of Products, Net Sales of Products (detailing all deductions), any deductions pursuant to Sections 5.2.5(b) and 5.2.5(c), and all Royalty Payments payable to NOVARTIS for the applicable Calendar Quarter (including any foreign exchange rates used), in all cases denominated in US Dollars (for sales within the US) and the relevant local currency converted into US Dollars based on the then applicable European Central Bank conversion rate for such currency (for ex-US sales). |
| 5.2.6 | Sharing of Sublicense Income. |
| (a) | LICENSEE will pay to NOVARTIS a percentage of Sublicense Income received in any Calendar Quarter during the Term as set forth below. |
| (i) | [***] of Sublicense Income if the Sublicense is executed prior to the achievement of the Progress Milestone; or |
| (ii) | [***] of Sublicense Income if the Sublicense is executed after the Progress Milestone is met. |
23
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| (b) | Notwithstanding the foregoing, if any Sublicense includes a grant of rights to exploit products other than Products (in addition to a grant of rights to exploit Products) (a “Multi-Product Sublicense”), then, LICENSEE shall be entitled to allocate in good faith the consideration received for such Multi-Product Sublicense to the Sublicense of rights related to Products and the license of rights related to other products based on the relative value of such Sublicense rights to such other rights and, within [***] days after the execution of such Multi-Product Sublicense, LICENSEE will provide written notice to NOVARTIS (the “Allocation Notice”) of such proposed allocation of consideration, which will included an analysis of its methodology and data used in connection with the Proposed Allocation (the “Proposed Allocation”). LICENSEE and NOVARTIS will discuss such Proposed Allocation in good faith. NOVARTIS may, within [***] days after delivery of such notice, or such longer period as Novartis may reasonably request (the “Allocation Notice Period”), object to such Proposed Allocation (an “Allocation Dispute”). In the event of any such Allocation Dispute, the Parties will resolve such dispute pursuant to Section 5.2.6(c); provided that, any payment obligation of any Sublicense Income due under Section 5.2.6 under such Multi-Product Sublicense shall be tolled until the resolution of such dispute pursuant to Section 5.2.6(c); provided further that, upon the resolution of such dispute, LICENSEE shall pay NOVARTIS for any Sublicense Income due to NOVARTIS under such Multi-Product Sublicense by the later of: (i) [***] days after the resolution of such dispute; or (ii) the date which such payment is due under this Agreement pursuant to Section 5.2.6(d). For the avoidance of doubt, if NOVARTIS does not object to the Proposed Allocation within the Allocation Notice Period, then LICSENSEE’s Proposed Allocation shall be deemed to be the allocation under this Agreement with respect to Sublicense Income received under such Multi-Product Sublicense. For the avoidance of doubt, the portion of any consideration under a Multi-Product Sublicense that is allocated to non-Products pursuant to this Section 5.2.6(b) shall not be considered Sublicense Income. |
| (c) | In the event of an Allocation Dispute, the Parties will work in good faith to resolve the disagreement. If the Parties are unable to reach a mutually acceptable resolution of any such dispute within [***] Business Days, such dispute will be resolved by submitting such dispute to a single neutral and independent arbitrator who is knowledgeable about the industry and the subject matter at issue in the dispute (the “Allocation Arbitrator”). The Parties shall mutually agree on the Allocation Arbitrator. Within [***] Business Days after the selection of the Allocation Arbitrator, each Party shall submit its proposed allocation of the |
24
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| consideration received for such Multi-Product Sublicense to the Sublicense of rights related to Products and the license of rights related to other products based on the relative value of such Sublicense rights to such other rights, along with any documentation, data or materials supporting such allocation that such Party desires to submit; provided that, LICENSEE may only submit such documentation, data or materials to the Allocation Arbitrator to the extent that LICENSEE previously provided such materials to NOVARTIS pursuant to Section 5.2.6(b) (each, a “Proposed Dispute Allocation”), to the Allocation Arbitrator. The Allocation Arbitrator shall render its decision within [***] Business Days after receipt of the Proposed Dispute Allocations from the Parties. The Allocation Arbitrator shall choose the Proposed Dispute Allocation submitted by either LICENSEE or NOVARTIS and may not modify such chosen Proposed Dispute Allocation in any way. The decision of the Allocation Arbitrator shall be final and binding upon the Parties, and the fees and expenses of the Allocation Arbitrator shall be borne by the Party whose Proposed Dispute Allocation is not chosen by the Allocation Arbitrator. |
| (d) | LICENSEE shall pay amounts due to NOVARTIS under this Section 5.2.6 within [***] days of the end of each Calendar Quarter, and at such time will deliver to NOVARTIS a report setting forth for such Calendar Quarter all Sublicense Income received by LICENSEE and the portion of any Sublicense Income due to NOVARTIS under this Section 5.2.6. |
| 5.3 | Payment Method. |
| 5.3.1 | Currency. With respect to Net Sales invoiced in U.S. dollars, the Net Sales and the amounts due for Royalty Payments under Section 5.2.5 will be expressed in U.S. dollars. With respect to Net Sales invoiced in a currency other than U.S. dollars, such conversion shall be made using the conversion methodology described in Section 5.2.5(e). |
| 5.3.2 | Method of Payment. All payments to a Party shall be made by wire transfer in U.S. Dollars to the credit of such bank account as may be designated by such Party to the other Party in writing. Any payment which falls due on a date which is not a Business Day may be made on the next succeeding Business Day. |
| 5.4 | Taxes. |
| 5.4.1 | VAT. Notwithstanding anything to the contrary in this Agreement, this Section 5.4.1 shall apply with respect to value added tax or any similar tax (“VAT”). All payments are exclusive of VAT. If any VAT is required in |
25
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| respect of any payments made under this Agreement under Applicable Law, LICENSEE shall pay VAT at the applicable rate in respect of any such payments following the receipt of a valid VAT invoice in the appropriate form issued by NOVARTIS in respect of those payments. NOVARTIS shall issue invoices for all amounts payable under this Agreement consistent with the applicable requirements for VAT. Any VAT included in an invoice will be payable by LICENSEE to NOVARTIS within [***] days after the receipt by LICENSEE of the applicable valid invoice relating to that VAT payment. LICENSEE shall not be responsible for any penalties or interest resulting from the failure by NOVARTIS to collect (if not included on a valid VAT invoice) or remit any such VAT. NOVARTIS and LICENSEE shall reasonably cooperate to eliminate or minimize the amount of any such VAT imposed on the transactions contemplated in this Agreement. If the VAT originally paid or otherwise borne by the LICENSEE is in whole or in part subsequently determined not to have been chargeable, all necessary steps will be taken by NOVARTIS to receive a refund of such undue VAT from the applicable taxing authority or other fiscal authority and any amount of VAT repaid by such taxing authority or other fiscal authority will be transferred to LICENSEE within [***] days of receipt. |
| 5.4.2 | Tax Cooperation. To the extent LICENSEE is required to deduct and withhold taxes on any payments to NOVARTIS, LICENSEE shall pay the amounts of such taxes to the proper Governmental Authority in a timely manner and promptly transmit to NOVARTIS an official tax certificate or other evidence of such withholding sufficient to enable NOVARTIS to claim such payments of taxes. NOVARTIS shall provide to LICENSEE any tax forms that may be reasonably necessary in order for LICENSEE not to withhold tax or to withhold tax at a reduced rate under an applicable bilateral income tax treaty. Each party shall provide the other with reasonable assistance to enable the recovery, as permitted by law, of withholding taxes, VAT, or similar obligations resulting from payments made under this Agreement, such recovery to be for the benefit of the party bearing such withholding tax or VAT. |
| 5.4.3 | Tax Forms. The parties agree to cooperate and produce on a timely basis any tax forms or reports reasonably requested by the other Party in connection with any payment made by the LICENSEE to NOVARTIS under this Agreement. |
| 6. | RECORDS; AUDIT RIGHTS |
| 6.1 | Relevant Records. |
| 6.1.1 | Relevant Records. LICENSEE shall keep, and will cause each of its Affiliates or Sublicensees, as applicable, to keep, accurate books and records of accounting for the purpose of calculating all payments due to |
26
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| NOVARTIS under Section 5 (such payments, collectively the “Fees” and such books and records, collectively the “Relevant Records”). Such Relevant Records will be kept by LICENSEE or such Affiliate or Sublicensee for [***] following the end of the Calendar Year to which each Relevant Record will pertain. |
| 6.1.2 | Audit Request. At the request of NOVARTIS, LICENSEE and its Affiliates and Sublicensees shall permit NOVARTIS to engage an independent certified public accounting firm reasonably acceptable to LICENSEE, during normal business hours not more than once a year and upon reasonable notice, to audit the Relevant Records in order to verify, with respect to any Calendar Year, the correctness or completeness of any payment made under this Agreement. The independent certified public accounting firm shall disclose to NOVARTIS only the amounts which the independent certified public accounting firm believes to be inaccurate or due and payable hereunder to NOVARTIS, shall provide a copy of same to LICENSEE and its Affiliates and Sublicensees (as applicable), and shall disclose no other information revealed in such audit. Any and all records of LICENSEE and its Affiliates and Sublicensees examined by such independent certified public accounting firm shall be deemed LICENSEE’s Confidential Information, which may not be disclosed by said independent certified public accounting firm to any Third Party or (except for the information expressly sought to be confirmed by NOVARTIS as set forth in this Section 6.1.2) to NOVARTIS. |
| 6.1.3 | Audit Fees and Expenses. NOVARTIS shall bear any and all fees and expenses incurred by it in connection with any such audit of the Relevant Records; provided, however, in the event an audit reveals an underpayment by LICENSEE of more than [***] as to the period subject to the audit, LICENSEE shall reimburse NOVARTIS for any reasonable and documented out-of-pocket costs and expenses of the audit within [***] after receiving invoices thereof. |
| 6.1.4 | Payment of Deficiency; Dispute. If such audit or, if the Parties dispute the findings of such audit, an independent accounting firm as described below, concludes that additional payments were owed or that excess payments were made during such period, or if the Parties otherwise agree that additional payments were owed or that excess payments were made during such period, LICENSEE will pay the additional amounts due or NOVARTIS will reimburse such excess payments, within sixty (60) days after the date on which a written report of such audit by the independent accounting firm pursuant to Section 6.1.2 or the report of the final determination of such independent accounting firm is delivered to the Parties pursuant to this Section 6.1.4 or on which the Parties reach such agreement, as the case may be plus interest computed at the 3 month US LIBOR rate as published in the Wall Street Journal [***]). In the event of a dispute regarding any audit under Section 6.1.2, the Parties will work in |
27
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| good faith to resolve the disagreement. If the Parties are unable to reach a mutually acceptable resolution of any such dispute within thirty (30) days, such dispute will be resolved by submitting such dispute to an independent accounting firm mutually agreeable to both Parties, which firm shall render its decision within sixty (60) days after submission of the dispute to such firm. The decision of such firm shall be final and binding upon the Parties, and the costs of such proceeding shall be borne by the Party whose position is such dispute is not affirmed by such firm, or, if such firm does not affirm either Party’s position in such dispute, such costs shall be borne between the Parties in such manner as such firm shall determine. |
| 6.1.5 | Confidentiality. NOVARTIS shall treat all information subject to review under this Section 6.1 in accordance with the confidentiality provisions of Section 9 and the Parties will cause any auditor to enter into a reasonably acceptable confidentiality agreement with LICENSEE obligating such firm to retain all such financial information in confidence pursuant to such confidentiality agreement. |
| 7. | INTELLECTUAL PROPERTY RIGHTS |
| 7.1 | Pre-existing IP. Subject only to the rights expressly granted to the other Party under this Agreement, each Party shall retain all rights, title and interests in and to any Intellectual Property Rights that are owned, licensed or Sublicensed by such Party prior to or independent of this Agreement. |
| 7.2 | Developed IP. LICENSEE shall own all rights, title and interests in and to any Intellectual Property Rights that are both: (a) related to a Compound or Product; and (b) conceived solely by LICENSEE, its Affiliates or Sublicensees following the Effective Date (collectively, “Developed IP”). |
| 7.3 | Patent Prosecution and Maintenance of Licensed Patent Rights. |
| 7.3.1 | Prosecution and Maintenance of Licensed Patent Rights. NOVARTIS shall be responsible for filing, prosecuting (including in connection with any reexaminations, oppositions and the like) and maintaining the Licensed Patent Rights in the Territory. NOVARTIS shall be responsible for all costs and expenses in connection with such filing, prosecution and maintenance, provided that if NOVARTIS provides LICENSEE with a notice of intent to abandon, or not file a patent application included in, any of the Licensed Patent Rights at least sixty (60) days in advance of the relevant deadline: (a) NOVARTIS shall no longer be responsible for such costs and expenses relating to filing, prosecuting and maintaining (as applicable) such Licensed Patent Right; (b) LICENSEE may, or may allow a Third Party to, file, prosecute and maintain (in its sole discretion), at its sole cost, such Licensed Patent Right; and (c) upon LICENSEE’s request, NOVARTIS shall promptly provide all files related to filing, prosecuting and maintaining such Licensed Patent Right to counsel designated by LICENSEE. |
28
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 7.3.2 | Cooperation. NOVARTIS shall provide LICENSEE with material correspondence with the relevant patent offices pertaining to NOVARTIS’ prosecution of the Licensed Patent Rights, to the extent that any activities could reasonably relate to the Field or any Compound or Product. On at least a yearly basis, NOVARTIS shall provide to LICENSEE a report detailing the status of all Licensed Patent Rights, including any patent term extensions, and the anticipated expiration dates of any issued Patents. NOVARTIS shall provide LICENSEE a reasonable opportunity to review and comment on proposed submissions to any patent office with respect to the Licensed Patent Rights prior to submission, and NOVARTIS shall consider LICENSEE’s comments with respect to such submissions in good faith or, to the extent that TSRI is prosecuting any of the Licensed Patent Rights pursuant to the TSRI Agreement, NOVARTIS shall provide LICENSEE’s comments to TSRI. |
| 8. | ACTUAL OR THREATENED INFRINGEMENT, DISCLOSURE OR MISAPPROPRIATION. |
| 8.1 | Notification. Each Party shall promptly notify the other Party in writing of its becoming aware of (a) any actual or threatened infringement, misappropriation or other violation or challenge to the validity, scope or enforceability by a Third Party of any Licensed Technology in the Field (“Third Party Infringement”); or (b) initiation by a Third Party of an opposition proceeding against any Licensed Patent Rights, or initiation by LICENSEE of an opposition against a Third Party or any allegation by a Third Party that Intellectual Property Rights owned by it is infringed, misappropriated or violated by the Development, Commercialization or Use of any Compound or Product (“Defense Action”). |
| 8.2 | Third Party Infringements in the Field. |
| 8.2.1 | NOVARTIS First Right to Enforce. On a case by case basis), NOVARTIS will have the first right (but not the obligation) to control enforcement of the Licensed Technology against any Third Party Infringement, at its own expense, in its own name and, subject to this Section 8.2.1, under its own direction and control, or, subject to this Section 8.2.1, settle any such action or proceeding; provided, however, that NOVARTIS will have no right to grant a Sublicense, covenant not to xxx or other right with respect to a Compound or Product (including a Generic Equivalent) in the Field in the Territory without the prior written consent of LICENSEE); provided further that NOVARTIS shall consult with LICENSEE and shall consider LICENSEE’s recommendations regarding the proposed suit, action, or proceeding to the extent such Third Party Infringement relates to the Field. NOVARTIS shall not settle, stipulate to any facts or make any admission with respect to any Third |
29
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| Party Infringement without LICENSEE’s prior written consent (not to be unreasonably withheld or delayed) if such settlement, stipulation or admission would: (a) adversely affect the validity, enforceability or scope, or admit non-infringement, of any of the Licensed Technology with respect to the Field; or (b) give rise to liability of LICENSEE or its Affiliates. |
| 8.2.2 | LICENSEE shall have the right (but not the obligation) to control enforcement of the Licensed Technology against any Third Party Infringement if (a) such Third Party Infringement is relates solely to the Field; and (b) NOVARTIS provides LICENSEE with written notice that it is not exercising its right to control such enforcement, or (c) if NOVARTIS fails to initiate, or file the relevant response to (as applicable), a suit, action or proceeding with respect to such Third Party Infringement upon the earlier of: (i) expiration of the [***] day period following first receipt by either Party of notice from the other Party of such Third Party Infringement; or (ii) [***] days prior to the deadline for filing, or filing the applicable response to (as applicable), such suit, action or proceeding (including suits, actions or proceedings based on a Third Party’s filing pursuant to 42 USC 262). If necessary for LICENSEE to exercise its rights under this Section 8.2.2, and if requested by LICENSEE, NOVARTIS will, at LICENSEE’S expense, act on LICENSEE’S behalf and as LICENSEE’s agent under any enforcement right held by NOVARTIS under the TSRI Agreement and, in connection therewith, will act on LICENSEE’s instruction and undertake any actions requested by LICENSEE, including bringing any action in any court requested by LICENSEE in NOVARTIS’s name. |
| 8.2.3 | Cooperation. Except as provided in this Section 8.2.3, notwithstanding anything to the contrary herein, the Party that is not controlling the suit, action or proceeding pertaining to enforcement of the Licensed Technology against Third Party Infringement as described in this Section 8.2 may, at its sole discretion and expense (subject to Section 8.3), join as a party to such suit, action or proceeding, provided that such Party shall join as a party to such suit, action or proceeding upon the reasonable request and expense of the Party controlling such action if necessary for standing purposes. The Party that is not controlling such a suit, action or proceeding shall have the right to be represented by counsel (which shall act in an advisory capacity only, except for matters solely directed to such Party) of its own choice and at its own expense (subject to Section 8.3) in any such suit, action or proceeding. |
| 8.2.4 | Recoveries. Any and all recoveries resulting from a suit, action or proceeding relating to a claim of Third Party Infringement shall first be applied to reimburse each Party’s costs and expenses in connection with such suit, action or proceeding (such recoveries to be applied pro rata in accordance with the costs and expenses incurred by each Party, in the |
30
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| event that the amount of such recoveries is less than the total amount of all such costs and expenses), with any remaining recoveries (a) if such Third Party Infringement is occurring both within and outside the Field, then allocated in accordance with the award in such suit, action or proceeding if such award allocates recoveries based on recoveries within and outside the Field, and if not, then split on a 50:50 basis; (b) retained by NOVARTIS if such Third Party Infringement is occurring solely outside the Field; and (c) retained by LICENSEE if such Third Party Infringement is occurring solely within the Field (the “Remaining Recoveries”). Notwithstanding the foregoing, LICENSEE shall pay NOVARTIS Royalty Payments and Sales Milestone Payments in accordance with Section 5 on the Remaining Recoveries retained or received by LICENSEE as if such Remaining Recoveries retained or received by LICENSEE were Net Sales in the Calendar Quarter in which such Remaining Recoveries were retained or received. |
| 8.3 | Defense Actions. Upon LICENSEE’s request, NOVARTIS shall reasonably cooperate with LICENSEE, to the extent necessary to defend LICENSEE or its Affiliates or any Sublicensee of LICENSEE in a Defense Action related to LICENSEE’s or its Affiliates or Sublicensee’s Development, Commercialization or Use of any Compound or Product in the Field. LICENSEE shall have all authority with respect to any Defense Action, including the right to exclusive control of the defense of any such suit, action or proceeding and the exclusive right to compromise, litigate, settle or otherwise dispose of any such suit, action, or proceeding, provided that LICENSEE shall keep NOVARTIS timely informed of the proceedings and filings, and provide NOVARTIS with copies of all material communications, pertaining to each Defense Action, and LICENSEE shall not settle, stipulate to any facts or make any admission with respect to any Defense Action without NOVARTIS’ prior written consent (not to be unreasonably withheld or delayed) if such settlement, stipulation or admission would (a) adversely affect the validity, enforceability or scope, or admit infringement, of any of the Licensed Technology; (b) give rise to liability of NOVARTIS or its Affiliates; or (c) otherwise impair NOVARTIS’ or any of its Affiliates’ rights in any Licensed Technology or NOVARTIS’ or any of its Affiliates’ rights in this Agreement, including rights outside the Field. LICENSEE will reimburse NOVARTIS for its reasonable costs and expenses associated with compliance with this Section 8.3 arising from LICENSEE’s or its Affiliates or Sublicensee’s Development, Commercialization or Use of any Compound or Product in the Field. |
| 9. | CONFIDENTIALITY |
| 9.1 | Definition. “Confidential Information” means the terms and provisions of this Agreement and other proprietary information and data of a financial, commercial or technical nature, that the disclosing Party or any of its Affiliates or Sublicensees has supplied or otherwise made available to the other Party or its Affiliates or Sublicensees, which are disclosed in writing, orally, electronically or |
31
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| other form, either in connection with the discussions and negotiations pertaining to this Agreement or in the course of performing under this Agreement, which may include information or data that is marked or otherwise identified as confidential or that by its nature a reasonable person would understand to be confidential in connection with this Agreement. |
| 9.2 | Obligations. During the term of this Agreement and for seven (7) years thereafter (and, with respect to the TSRI IP, during the term of the TSRI Agreement and for three years thereafter), the receiving Party will (a) protect all Confidential Information of the disclosing Party against unauthorized disclosure to Third Parties and (b) not use or disclose the Confidential Information of the disclosing Party, except as permitted by or in furtherance of exercising rights or carrying out obligations hereunder or for internal legal, accounting or finance purposes; provided, however, that such time period will be extended if and for so long as the disclosing Party maintains the relevant Confidential Information as a trade secret under Applicable Law. The receiving Party shall treat all Confidential Information provided by the disclosing Party with the same degree of care as the receiving Party uses for its own similar information, but in no event less than a reasonable degree of care. The receiving Party may disclose the Confidential Information to its Affiliates and its and their Sublicensees, and their respective directors, officers, employees, subcontractors, consultants, attorneys, accountants, banks and investors (collectively, “Recipients”) who have a need-to-know such information for purposes related to this Agreement, provided that the receiving Party shall hold such Recipients to obligations of confidentiality with terms and conditions at least as restrictive as those set forth in this Agreement; and provided further that the receiving Party shall be liable for any action or inaction of its Representatives that would be considered a breach of this Section 9 if committed by the receiving Party. |
| 9.3 | Exceptions to Confidentiality. The obligations under this Section 9 shall not apply to any information to the extent the receiving Party can demonstrate by competent evidence that such information: |
| (a) | is (at the time of disclosure) or becomes (after the time of disclosure) known to the public or part of the public domain through no breach of this Agreement by the receiving Party or any Recipients to whom it disclosed such information; |
| (b) | was known to, or was otherwise in the possession of, the receiving Party prior to the time of disclosure by the disclosing Party, other than under an obligation of confidentiality; |
| (c) | is disclosed to the receiving Party on a non-confidential basis by a Third Party who is entitled to disclose it without breaching any confidentiality obligation to the disclosing Party; or |
32
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| (d) | is independently developed by or on behalf of the receiving Party or any of its Affiliates, as evidenced by its written records, without use or access to the Confidential Information. |
| 9.4 | Permitted Disclosures. |
| 9.4.1 | Compliance with Law. The restrictions set forth in this Section 9 shall not apply to any Confidential Information that the receiving Party is required to disclose under Applicable Laws or a court order or other governmental order or to enforce any Licensed Patent Rights under Section 8, provided that the receiving Party: (a) provides the disclosing Party with prompt notice of such disclosure requirement if legally permitted; (b) to the extent practical, affords the disclosing Party an opportunity to oppose or limit, or secure confidential treatment for such required disclosure; and (c) if the disclosing Party is unsuccessful in its efforts pursuant to subsection (b), discloses only that portion of the Confidential Information that the receiving Party is legally required to disclose as advised by the receiving Party’s legal counsel. |
| 9.4.2 | Other Permitted Disclosures. Notwithstanding the restrictions set forth in this Section 9, a Party may, without the prior consent of the other Party, disclose Confidential Information: |
| (a) | to Third Parties that have a legitimate need to know such information as part of such Party’s financing activities or in connection with any sale or other transfer of such Party’s business or assets, solely for the purpose of evaluating or carrying out an actual or potential investment, acquisition or other transaction, provided that the recipients of such information are bound by written confidentiality obligations consistent with those set forth in this Section 9, and provided further that such Party shall be liable for any action or inaction of such Third Parties that would be considered a breach of this Section 9 if committed by such Party; |
| (b) | for preparing, filing, prosecuting, or maintaining Patents as permitted by this Agreement; |
| (c) | for making regulatory filings for a Product that such Party has a license or right to develop hereunder in a given country or jurisdiction; |
| (d) | for prosecuting or defending litigation as permitted by this Agreement; |
| (e) | to an underwriter or placement agent or its counsel in connection with any offering by LICENSEE; or |
| (f) | to a permitted assignee of this Agreement. |
33
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 9.4.3 | LICENSEE Permitted Disclosures. Notwithstanding the restrictions set forth in this Section 9, in the event that LICENSEE wishes to enter into a Sublicense in accordance with Section 2.2, LICENSEE may disclose to a Third Party Confidential Information of NOVARTIS relating to the Products or Compounds in connection with any such proposed Sublicense, provided that LICENSEE shall hold such Third Parties to written obligations of confidentiality with terms and conditions at least as restrictive as those set forth in this Agreement. |
| 9.5 | Right to Injunctive Relief. Each Party agrees that breaches of this Section 9 may cause irreparable harm to the other Party and shall entitle such other Party, in addition to any other remedies available to it (subject to the terms of this Agreement), the right to seek injunctive relief enjoining such action. Both Parties agree to waive any requirement that the other post a bond or other security as a condition for obtaining any such relief. |
| 9.6 | Return or Destruction of Confidential Information. Upon expiration or termination of this Agreement, the receiving Party shall, and shall cause its Recipients to, destroy, delete or return (as requested by the disclosing Party) any Confidential Information of the disclosing Party, except for one copy which may be retained in its confidential files for archive purposes. |
| 10. | REPRESENTATIONS, WARRANTIES AND COVENANTS |
| 10.1 | Representations and Warranties by Each Party. Each Party represents and warrants to the other Party as of the Effective Date that: |
| (a) | it is a corporation duly organized, validly existing, and in good standing under the laws of its jurisdiction of formation; |
| (b) | it has full corporate power and authority to execute, deliver, and perform under this Agreement, and has taken all corporate action required by Applicable Laws and its organizational documents to authorize the execution and delivery of this Agreement and the consummation of the transactions contemplated by this Agreement, and the person or persons executing this Agreement on its behalf has been duly authorized to do; |
| (c) | this Agreement constitutes a valid and binding agreement enforceable against it in accordance with its terms; |
| (d) | all consents, approvals and authorizations from all Governmental Authorities or other Third Parties required to be obtained by such Party in connection with this Agreement have been obtained; |
| (e) | the execution and delivery of this Agreement and all other instruments and documents required to be executed pursuant to this Agreement, and the consummation of the transactions |
34
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| contemplated hereby do not and shall not: (i) conflict with or result in a breach of any provision of its organizational documents; (ii) result in a breach of any agreement to which it is a party that would impair the performance of its obligations hereunder; (iii) violate any Applicable Laws; or (iv) violate any order, writ, judgment, injunction, decree, determination or award of any court or Governmental Authority presently in effect applicable to it; and |
| (f) | there is no action, suit, proceeding or investigation pending or, to its knowledge, currently threatened in writing against or affecting it that questions the validity of this Agreement or the right of it to enter into this Agreement or consummate the transactions contemplated hereby and, to its knowledge, there is no basis for the foregoing. |
| 10.2 | Representations, Warranties and Covenants by NOVARTIS. |
| 10.2.1 | NOVARTIS represents and warrants to LICENSEE as of the Effective Date and covenants to LICENSEE as follows: |
| (a) | NOVARTIS owns and Controls the Licensed Patent Rights (other than the Licensed Patent Rights included in the TSRI IP) and the Licensed Know-How (other than the Licensed Patent Rights included in the TSRI IP), and is entitled to grant the licenses specified herein, and other than the Licensed Patent Rights and the Licensed Know-How included in the TSRI IP, all of the Licensed Patent Rights and Licensed Know-How is owned by NOVARTIS; |
| (b) | all Licensed Patent Rights existing on the Effective Date are identified on Schedule A; |
| (c) | NOVARTIS does not Control any Patents, other than those listed on Schedule A, a license to which is necessary to practice the licenses granted herein or to Use the Compounds or Products in the Field; |
| (d) | to the Knowledge of the Patent Associates, (a) the issued Licensed Patent Rights are valid and enforceable Patents and (b) no Patent registration within the issued Licensed Patent Rights is the subject of any pending interference, opposition, cancellation, or patent protest pursuant to 37 C.F.R. § 1.291; |
| (e) | NOVARTIS has not granted to any Third Party any rights or licenses under any of the Licensed Patent Rights or Licensed Know-How that would conflict with the licenses granted to LICENSEE hereunder, and it will not grant any such rights or licenses during the term of this Agreement; |
35
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| (f) | each issued Licensed Patent Right remains in full force and effect and all filing and renewal fees payable with respect to each Licensed Patent Right have been timely paid; |
| (g) | to the Knowledge of the Patent Associates, the Licensed Patent Rights and the Licensed Know-How are free and clear of (i) all mortgages, pledges, charges, liens, equities, security interests, or other encumbrances (other than, in the case of the TSRI IP, rights retained by the United States Government under Applicable Law), and (ii) any covenants that would conflict with or limit the scope of the licenses granted to LICENSEE hereunder; |
| (h) | other than with respect to the TSRI Agreement, NOVARTIS is not subject to any royalty or similar payment obligation to any Third Party with respect to the grant of rights to LICENSEE to practice the Licensed Technology; |
| (i) | to the Knowledge of the Patent Associates and to the Knowledge of the Novartis Deal Team, (i) LICENSEE’s Use of Compounds in the Field within the Territory or the practice of the Licensed Technology, as permitted by this Agreement, will not infringe any U.S. Patent owned or Controlled by a Third Party or misappropriate or otherwise violate the Intellectual Property Rights of a Third Party; provided, however, that reagents used in the manufacture protocols described in the Licensed Know-How have not been evaluated for commercial availability and, if implemented in the final commercial manufacture, may require a commercial license from a Third Party, and (ii) , NOVARTIS has not received any written notice from a Third Party, alleging that the Use of the Compounds or Product in the Field within the Territory infringes, misappropriates or otherwise violates the Intellectual Property Rights of a Third Party; |
| (j) | to the Knowledge of the Patent Associates and to the Knowledge of the Novartis Deal Team, there is no claim pending or threatened by NOVARTIS alleging that a Third Party is or was infringing, misappropriating or otherwise violating the Licensed Technology in the Field within the Territory, and, to the Knowledge of the Patent Associates and to the Knowledge of the Novartis Deal Team, there is no reasonable basis for any such allegation; |
| (k) | with respect to any pending applications included in the Licensed Patent Rights, such applications are being prosecuted in the respective patent offices in the countries in the Territory, in accordance with Applicable Law and the attorneys, agents and representatives of NOVARTIS and their respective Affiliates prosecuting such pending applications have submitted all known |
36
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| material prior art with respect to each pending and issued Licensed Patent Right to the U.S. Patent and Trademark Office or equivalent Governmental Authority in the jurisdiction in which such Patent is issued or pending, in each case as and when required to meet the duty to disclose information material to patentability as required under 37 C.F.R. 1.56 or to comply with analogous Applicable Law outside the United States requiring disclosure of references; |
| (l) | the Knowledge of the Patent Associates, NOVARTIS or its Affiliate has obtained from all inventors of each Patent included in the Licensed Patent Rights (other than those included in the TSRI IP) an assignment to NOVARTIS or its Affiliates, as applicable, of each such inventor’s entire rights, title and interests in and to such Licensed Patent Right, and to the Knowledge of the Patent Associates, no current officer, employee, agent, or consultant of Novartis or any of its Affiliates is in violation of any term of any such assignment agreement; |
| (m) | NOVARTIS has taken measures consistent with its ordinary practice to ensure that each Licensed Patent Right properly identifies each and every inventor of the claims thereof as determined in accordance with the laws of the jurisdiction in which such Licensed Patent Right is issued or pending; |
| (n) | NOVARTIS has taken measures consistent with its ordinary practice to protect the secrecy, confidentiality and value of all Licensed Know-How that constitutes trade secrets under Applicable Law (including requiring all employees, consultants and independent contractors to execute binding and enforceable confidentiality agreements and requiring all such employees, consultants and independent contractors to maintain the confidentiality of such Licensed Know-How) and, to the Knowledge of Novartis, such Licensed Know-How has not been used, disclosed to or discovered by any Third Party (other than Licensed Know-How included in the TSRI IP) except pursuant to such confidentiality agreements, and there has not been a breach by any party to such confidentiality agreements, and NOVARTIS will use reasonable efforts to maintain any such trade secrets as such in accordance with Applicable Law; |
| (o) | other than the Licensed Patent Rights included in the TSRI IP, the development of the Licensed Patent Rights were not funded in whole or in part by the government of the United States of America, are not a “subject invention” as that term is described in 35 U.S.C. Section 201(e) and are not otherwise subject to the provisions of the Xxxx-Xxxx Act; |
37
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| (p) | no event has occurred, and no condition or circumstance exists, that would reasonably be expected to (with or without the notice or lapse of time) constitute, or result in, a default under, a breach or violation of, or a failure to comply with any Applicable Law by NOVARTIS, its Affiliates, or its contract research organizations, except for those events, conditions or circumstances which individually or in the aggregate would not have a material adverse effect on the continued use of the Compounds, in the manner that the Compounds had been used by NOVARTIS prior to the Effective Date; |
| (q) | NOVARTIS, its Affiliates and, to the Knowledge of NOVARTIS, its contract research organizations, have generated, prepared, maintained and retained all documentation regarding the Development of the Product that is required to be maintained or retained pursuant to and in accordance with good laboratory and clinical (if applicable) practice and Applicable Law, and all such information is true, complete and correct, except for those failures that would not, individually or in the aggregate, have a material adverse effect on the continued use of the Compounds, in the manner that the Compounds had been used by NOVARTIS prior to the Effective Date; |
| (r) | to the Knowledge of Novartis, neither NOVARTIS nor any of its Affiliates, nor any of its or their respective officers, employees or agents, has (i) committed an act, (ii) made a statement or (iii) failed to act or make a statement, in any case ((i), (ii) or (iii)), that (A) would be or create an untrue statement of material fact or fraudulent statement to the FDA or any other Regulatory Authority with respect to the exploitation of the Compounds or the Product or (B) could reasonably be expected to provide a basis for the FDA to invoke its policy respecting “Fraud, Untrue Statements of Material Facts, Bribery and Illegal Gratuities”, set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto or any analogous laws or policies in the Territory, with respect the Exploitation of the Compounds or the Product; |
| (s) | NOVARTIS has filed or caused to be filed with the relevant Regulatory Authorities in the Territory, in each case to the extent required by Applicable Law to be filed by or on behalf of NOVARTIS, all material notices, amendments and annual reports, as well as adverse event reports for the Compounds; |
| (t) | there is no pending action or, to the Knowledge of Novartis, action threatened in writing by relevant Regulatory Authorities to place a clinical hold order on, or otherwise terminate or suspend, any clinical trial authorization for the Product; |
38
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| (u) | NOVARTIS will (i) maintain the TSRI Agreement in full force and effect until the last to expire Patent rights included in that agreement, (ii) not modify or amend the TSRI Agreement in any manner that would be inconsistent with or otherwise contravene the rights and licenses granted to LICENSEE under this Agreement and (iii) will promptly notify LICENSEE of any notice of default or termination received by NOVARTIS with respect to the TSRI Agreement, and in the event of any such default, upon LICENSEE’s request, NOVARTIS shall allow LICENSEE to cure any such default on behalf of NOVARTIS, and LICENSEE may offset any costs or expenses for such cure to any payments due to NOVARTIS under this Agreement; |
| (v) | other than the HemOnc IND and the IMD IND, NOVARTIS and its Affiliates do not Control any other Regulatory Filings; and |
| (w) | NOVARTIS has not created, conceived of, or reduced to practice any Jointly Developed Technology (as defined under the TSRI Agreement). |
| 10.3 | No Other Warranties. EXCEPT AS EXPRESSLY STATED IN THIS SECTION 10, NEITHER PARTY MAKES ANY REPRESENTATIONS OR EXTENDS ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, STATUTORY OR OTHERWISE, INCLUDING BUT NOT LIMITED TO WARRANTIES OF TITLE, NON-INFRINGEMENT, VALIDITY, ENFORCEABILITY, MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. |
| 10.4 | Compliance with Applicable Laws. In the performance of its obligations under this Agreement, each Party shall, and shall ensure all Third Parties that it engages, comply with all Applicable Laws. |
| 11. | INDEMNIFICATION |
| 11.1 | Indemnification by LICENSEE. LICENSEE agrees to indemnify, hold harmless and defend NOVARTIS, its Affiliates, subcontractors and Sublicensees, and their respective officers, directors and employees (collectively, “NOVARTIS Indemnitees”), from and against any Losses in connection with Claims arising or resulting from: (a) the manufacture, Development, or Use of the Compound, including the Use of the Compound in Development and Commercialization of Products by LICENSEE Indemnitees, (b) the Development of a Product by, on behalf of or under grant of rights from LICENSEE Indemnitees; (c) the Commercialization of a Product by, on behalf of or under grant of rights from LICENSEE Indemnitees; (d) the gross negligence or wrongful intentional acts or omissions of LICENSEE Indemnities in connection with this Agreement; or (e) any breach by LICENSEE of any representation, warranty or covenant as set forth in this Agreement. As used herein, “Claims” means collectively, any and |
39
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| all Third Party demands, claims, actions and proceedings (whether criminal or civil, in contract, tort or otherwise) for Losses. As used herein, “Losses” means any losses, damages, liabilities, costs and expenses (including reasonable attorneys’ fees). |
| 11.2 | Indemnification by NOVARTIS. NOVARTIS agrees to indemnify, hold harmless and defend LICENSEE, its Affiliates, subcontractors or Sublicensees and their respective officers, directors and employees (collectively, “LICENSEE Indemnitees”) from and against any Claims arising or resulting from: (a) the Development and other Use of the Compounds, including the use of Compounds in the manufacture of therapeutic products, outside of the Field by the NOVARTIS Indemnitees; (b) the gross negligence or wrongful intentional acts or omissions of NOVARTIS Indemnitees in connection with this Agreement; (c) any breach by NOVARTIS of any representation, warranty, obligation or covenant as set forth in this Agreement; or (d) the Use of any Gene-Edited/-Modified HSC Improvement by NOVARTIS Indemnitees. |
| 11.3 | Indemnification Procedures. |
| 11.3.1 | A Party that intends to claim indemnification under this Section 11 (the “Indemnified Party”) shall provide written notice to the indemnifying Party (the “Indemnifying Party”) of any Claim as soon as reasonably possible, and in any event no later than within thirty (30) days after the Indemnified Party has actual knowledge of such claim, demand or action (an “Indemnification Claim Notice”); provided, however, that, if the Indemnified Party fails to promptly notify the Indemnifying Party pursuant to the foregoing clause, the Indemnifying Party will only be relieved of its indemnification obligation to the extent materially prejudiced by such failure. Such Indemnification Claim Notice shall include a description of the Claim and the nature and amount of any Losses (to the extent that the nature and amount of such Losses are known at such time). Together with the Indemnification Claim Notice, the Indemnified Party shall furnish promptly to the Indemnifying Party copies of all notices and documents (including court papers) received by any Indemnified Party in connection with the Claim. |
| 11.3.2 | At its option, the Indemnifying Party may assume the defense and have exclusive control, at its own expense, of any Claim for which indemnity is being sought by giving written notice to the Indemnified Party within thirty (30) days after receipt of the notice of the Claim, provided that (i) it agrees to indemnify the Indemnified Party from and against all Losses the Indemnified Party may suffer arising out of the Claim; (ii) the Claim involves only money damages and does not seek an injunction or other equitable relief against the Indemnified Party; (iii) the Claim does not relate to any criminal or a regulatory enforcement proceeding; and (iv) the Indemnifying Party conducts the defense of the Claim diligently. The Indemnified Party will provide the Indemnifying Party with reasonable |
40
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| assistance, at the Indemnifying Party’s reasonable expense, in the investigation, preparation, defense, and settlement or voluntary disposition of any such claim, demand or action. The Indemnified Party may participate in and monitor such defense with counsel of its own choosing at its sole expense. The Indemnifying Party will have the right to assume and conduct the defense of the Claim with counsel of its choice. The Indemnifying Party will not settle any Claim without the prior written consent of the Indemnified Party, not to be unreasonably withheld, conditioned or delayed, unless the settlement (i) provides for the payment by the Indemnifying Party of money as sole relief for the claimant, (ii) results in the full and general release of the Indemnified Party from all liabilities arising or relating to, or in connection with, the Claim; and (iii) involves no finding or admission of any violation of Applicable Law or the rights of any Person and shall have no effect on any other claims that may be made against the Indemnified Party. |
| 11.3.3 | If the Indemnifying Party does not assume and conduct the defense of the Claim as provided in Section 11.3.2, (i) the Indemnified Party may defend against, and consent to the entry of any judgment or enter into any settlement with respect to the Claim in any manner the Indemnified Party may deem reasonably appropriate (and the Indemnified Party need not consult with, or obtain any consent from, the Indemnifying Party in connection therewith), and (ii) the Indemnified Party reserves any right it may have under this Section 11 to obtain indemnification from the Indemnifying Party for Losses. |
| 12. | LIMITATION OF LIABILITY. EXCEPT FOR A BREACH OF SECTION 9 OR OBLIGATIONS ARISING UNDER SECTION 11, NEITHER PARTY SHALL BE LIABLE FOR ANY INDIRECT OR CONSEQUENTIAL, SPECIAL, EXEMPLARY OR PUNITIVE DAMAGES, INCLUDING DAMAGES FOR LOST PROFITS OR LOST REVENUES REGARDLESS OF WHETHER IT HAS BEEN INFORMED OF THE POSSIBILITY OR LIKELIHOOD OF SUCH DAMAGES OR THE TYPE OF CLAIM, CONTRACT OR TORT (INCLUDING NEGLIGENCE). |
| 13. | TERM; TERMINATION |
| 13.1 | Term. The term of this Agreement shall commence as of the Effective Date and, unless earlier terminated as expressly provided herein, shall expire upon the last-to-expire Royalty Term (the “Term”). |
| 13.2 | Termination for Cause. |
| 13.2.1 | Each Party shall have the right, without prejudice to any other remedies available to it at law or in equity, to terminate this Agreement in the event the other Party breaches any of its material obligations hereunder or under the Subscription Agreement and fails to cure such breach within [***] |
41
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| days of receiving notice thereof (the “Notice Period”) by the other Party; provided, however, that if such breach (i) is not a payment breach; (ii) is capable of being cured, but cannot be cured within the Notice Period, and (iii) the breaching Party initiates good faith actions to cure such breach within the Notice Period and thereafter diligently pursues such actions, the period to cure such breach shall be extended so long as such good faith actions are being diligently pursued by the breaching Party, up to an additional [***] days. Any termination by a Party under this Section 13.2.1 shall be without prejudice to any damages or other legal or equitable remedies to which it may be entitled from the other Party. If either Party initiates a dispute resolution procedure under Section 15 during the Notice Period to resolve the dispute for which termination is being sought and is diligently pursuing such procedure, the cure period set forth in this Section 13.2.1 shall be tolled and the termination shall become effective (i) with respect to any breach that is capable of being cured, if the breaching Party does not implement the remedy determined through such dispute resolution procedure for such breach within the timeframe established through such dispute resolution procedure or (ii) with respect to any breach that is not capable of being cured, upon the final resolution of the dispute if the resolution includes the grant of the terminating Party’s request to terminate. |
| 13.2.2 | Notwithstanding the foregoing, without limiting any other remedies that LICENSEE may have under this Agreement, if LICENSEE is entitled to terminate this Agreement pursuant to Section 13.2.1, then, rather than terminating this Agreement, LICENSEE may elect, at its sole discretion, by notice in writing to NOVARTIS, to modify this Agreement as follows: |
| (a) | LICENSEE shall continue to pay Milestone Payments pursuant to Section 5.2.1 and Royalty Payments pursuant to Section 5.2.5 pursuant to the terms of the Agreement, but in an amount equal to [***] of the amount otherwise payable by LICENSEE under this Agreement (after applying any deductions or credits to which LICENSEE is entitled in accordance with the terms of this Agreement). |
| (b) | All other rights and obligations of the Parties’ under this Agreement shall remain in effect. |
| 13.3 | Termination by LICENSEE. LICENSEE may terminate this Agreement at will on a Product-by-Product and country-by-country basis, or in its entirety, in its sole discretion, on not less than ninety (90) days prior written notice to NOVARTIS. |
| 13.4 | Termination for a Bankruptcy Event. Each Party shall have the right to terminate this Agreement in the event of a Bankruptcy Event with respect to the other Party. “Bankruptcy Event” means the occurrence of any of the following: (a) the institution of any bankruptcy, receivership, insolvency, reorganization or |
42
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| other similar proceedings by or against a Party under any bankruptcy, insolvency, or other similar law now or hereinafter in effect, including any section or chapter of the United States Bankruptcy Code, as amended or under any similar laws or statutes of the United States or any state thereof (the “Bankruptcy Code”), where in the case of involuntary proceedings such proceedings have not been dismissed or discharged within ninety (90) days after they are instituted; (b) the insolvency or making of an assignment for the benefit of creditors or the admittance by a Party of any involuntary debts as they mature; (c) the institution of any reorganization, arrangement or other readjustment of debt plan of a Party not involving the Bankruptcy Code; (d) appointment of a receiver for all or substantially all of a Party’s assets; or (e) any corporate action taken by the board of directors of a Party in furtherance of any of the foregoing actions. |
| 13.5 | Bankruptcy. If this Agreement is rejected by a Party as a debtor under Section 365 of the Bankruptcy Code, then, notwithstanding anything else in this Agreement to the contrary, all licenses and rights to licenses granted under or pursuant to this Agreement by the Party in bankruptcy to the other Party are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the Bankruptcy Code, or comparable provision of applicable bankruptcy or insolvency laws, licenses of rights to “intellectual property” as defined under Section 101(35A) of the Bankruptcy Code, or comparable provision of applicable bankruptcy or insolvency laws. The Parties agree that a Party that is a licensee of such rights under this Agreement will retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code or comparable provision of applicable bankruptcy or insolvency laws. The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against a Party to this Agreement under the U.S. Bankruptcy Code or comparable provision of applicable bankruptcy or insolvency laws, the other Party will be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, and same, if not already in its possession, will be promptly delivered to it (a) upon any such commencement of a bankruptcy or insolvency proceeding upon its written request therefor, unless the bankrupt Party elects to continue to perform all of its obligations under this Agreement, or (b) if not delivered under (a) above, following the rejection of this Agreement by or on behalf of the bankrupt Party upon written request therefor by the other Party. Whenever the bankrupt Party or any of its successors or assigns provides to the other Party any of the intellectual property licensed hereunder (or any embodiment thereof) pursuant to this Section 13.5, the other Party shall have the right to perform the bankrupt Party’s obligations hereunder with respect to such intellectual property, but neither such provision nor such performance by the other Party shall release the bankrupt Party from liability resulting from rejection of the license or the failure to perform such obligations. The bankrupt Party shall not interfere with the other Party’s rights under this Agreement, or any agreement supplemental hereto, to such intellectual property (including such embodiments), including any right to obtain such intellectual property (or such embodiments) from another entity, to the extent provided in Section 365(n) of the U.S. Bankruptcy Code. All rights, powers and |
43
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| remedies of the non-subject Party provided herein are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at law or in equity (including the U.S. Bankruptcy Code) in the event of the commencement of an action under the U.S. Bankruptcy Code with respect to the bankrupt Party. The Parties agree that they intend the following rights to extend to the maximum extent permitted by law, and to be enforceable under U.S. Bankruptcy Code Section 365(n): (x) the right of access to any intellectual property (including all embodiments thereof) of the bankrupt Party, or any Third Party with whom the bankrupt Party contracts to perform an obligation of the bankrupt Party under this Agreement; and (y) the right to contract directly with any Third Party to complete the contracted work. |
| 13.6 | Effect of Termination or Expiration. |
| 13.6.1 | Upon termination or expiration of this Agreement, LICENSEE shall pay to NOVARTIS all Fees or other amounts due to NOVARTIS as of the effective date of termination or expiration within [***] days following the effective date of termination or expiration. |
| 13.6.2 | Upon expiration of this Agreement pursuant to Section 13.1 (but not upon termination of this Agreement), NOVARTIS hereby grants to LICENSEE a royalty-free right and license to Use the Licensed Know-How to Use Compounds and Products within the Territory. |
| 13.6.3 | Subject to Section 13.6.4(e), upon termination of this Agreement, (i) all licenses granted by NOVARTIS to LICENSEE will terminate; and (ii) LICENSEE shall have the right to sell its remaining inventory of Products following the termination of this Agreement so long as LICENSEE has fully paid, and continues to fully pay when due, any and all Fees owed to NOVARTIS. |
| 13.6.4 | Upon termination of this Agreement: |
| (a) | LICENSEE hereby grants to NOVARTIS a non-exclusive, fully paid-up, royalty-free, worldwide, perpetual and irrevocable license, with the right to sublicense, to Use any and all Developed IP and any other Intellectual Property Rights Controlled by LICENSEE that LICENSEE actually used in the Development, manufacture or Commercialization of the Product as the Product exists at the time of termination (“collectively, “Reversion IP”), solely for the Development and Commercialization of the Products provided however, that if any such Reversion IP is in-licensed from a Third Party and subject to payment and other applicable obligations to such Third Party, LICENSEE shall promptly disclose such obligations to NOVARTIS in writing and such Reversion IP shall be subject to the license granted in this Section 13.6.4(a) only if NOVARTIS agrees in writing to reimburse LICENSEE for all |
44
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| amounts owed to such Third Party as a result of NOVARTIS’ exercise of such license and comply with all obligations under any such Third Party license that are applicable to LICENSEE as a Sublicensee thereunder, and thereafter so reimburses LICENSEE and complies with such terms; |
| (b) | to the extent permitted by applicable Regulatory Authorities, LICENSEE shall: (i) transfer to NOVARTIS all Regulatory Filings and Regulatory Approvals held by LICENSEE that are solely related to the Product; and (ii) to the extent subsection (i) is not permitted by the applicable Regulatory Authority and with respect to Regulatory Filings held by LICENSEE that are not solely related to the Product, permit NOVARTIS to cross-reference and rely upon any Regulatory Approvals and Regulatory Filings filed by LICENSEE with respect to the Product solely to Use the Products; |
| (c) | LICENSEE, if requested in writing by NOVARTIS, shall provide any and all material correspondence with the relevant patent offices pertaining to the LICENSEE’s prosecution of the Licensed Patent Rights to the extent not previously provided to NOVARTIS during the course of the Agreement; |
| (d) | effective as of the date of termination, LICENSEE hereby grants to NOVARTIS a fully paid-up, royalty-free, worldwide, transferable, sublicensable, perpetual and irrevocable license to use the Trademarks Controlled by LICENSEE solely identifying a Product for the purpose of Commercializing the Products; |
| (e) | LICENSEE will responsibly wind-down, in accordance with accepted pharmaceutical industry norms and ethical practices, any on-going clinical studies of Products for which it has responsibility hereunder in which patient dosing has commenced or, if reasonably practicable and requested by NOVARTIS, allow NOVARTIS or its Affiliates or a Third Party that is designated in writing by NOVARTIS (“Designated Affiliate/Third Party”) to complete such trials (and then assign to NOVARTIS all related Regulatory Filings, Regulatory Approvals, and investigator and other agreements relating to such studies). LICENSEE shall be responsible for any Development costs associated with such wind-down; provided that NOVARTIS shall pay all Development costs incurred by either Party to complete such studies should NOVARTIS request that such studies be completed. During any such winding down of ongoing trials, LICENSEE shall provide such knowledge transfer and other training to NOVARTIS or its Designated Affiliate/Third Party as reasonably necessary for NOVARTIS or the Designated Affiliate/Third Party to continue |
45
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| such trial. In connection with such transfer, LICENSEE shall, at NOVARTIS’ option: [***]. As used herein, “LICENSEE Inventory” means all components and works in process produced or held by LICENSEE with respect to the manufacture of Products; and |
| (f) | any Sublicense shall, at Sublicensee’s option, survive any termination of this Agreement, provided that the Sublicensee is not in material breach of any of its obligations under such Sublicense such that LICENSEE would have a right to terminate such Sublicense. In connection with the foregoing survival, at the request of Sublicensee, NOVARTIS shall enter into a direct license with the Sublicensee on substantially the same terms as the Sublicense; provided that the Sublicense provides for consideration to NOVARTIS that is not less than the amount set forth in this Agreement; and provided further that, notwithstanding anything in this Agreement to the contrary, and whether or not NOVARTIS enters into a direct license with a Sublicensee pursuant to this Section 13.6.4(f), NOVARTIS shall not be required to undertake obligations in addition to those required by this Agreement, and that NOVARTIS’ rights under such direct license shall be consistent with its rights under this Agreement, taking into account the scope of the license granted under such direct license. |
| Notwithstanding the foregoing in this Section 13.6.4, in the event of termination by LICENSEE due to NOVARTIS’ material breach under Section 13.2.1, then LICENSEE shall have no obligation whatsoever to grant the licenses or perform any of the actions set forth in Sections 13.6.4(a), (b) and (d) unless and until NOVARTIS makes a payment to LICENSEE equal to the actual amount of Development costs and expenses incurred by LICENSEE and its Affiliates prior to the date of such termination. |
| 13.7 | Survival. The expiration of the Term or any termination of this Agreement for any reason will be without prejudice to any rights that have accrued to the benefit of any Party prior to such expiration or termination, and any such expiration or termination of this Agreement shall not relieve the Parties of any obligation accruing hereunder prior to such expiration or termination. Without limiting the foregoing, the provisions of Sections 1 (Definitions), 2.2 (Cross License to Licensed Patent Rights Improvements), 5.2 (Milestones and Royalty Payments), 5.3 (Payment Method) (to the extent payments are due hereunder), 5.4 (Taxes), 6 (Records; Audit Rights), 7.1 (Pre-existing IP), 7.2 (Developed IP), 9 (Confidentiality), 11 (Indemnification), 12 (Limitation of Liability), 13.6 (Effect of Termination or Expiration), 14 (Publicity and Publications), 16 (Dispute Resolution) and 17 (General Provisions) shall survive expiration or termination of this Agreement. |
46
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 13.8 | Termination Not Sole Remedy. Termination is not the sole remedy under this Agreement and, whether or not termination is effected and notwithstanding anything contained in this Agreement to the contrary, all other remedies will remain available except as agreed to otherwise herein. For the avoidance of doubt, nothing in this Agreement shall obligate a Party to terminate this Agreement in the event that the other Party breaches any obligation of this Agreement, and failure to terminate this Agreement shall not prohibit or modify the recovery of damages. |
| 14. | PUBLICITY AND PUBLICATIONS |
| 14.1 | Publicity and Publications. |
| 14.1.1 | Use of Trademarks. Subject to NOVARTIS’ rights pursuant to Section 13.6.4(d), neither Party (nor any of its Affiliates or agents) shall use the Trademarks of the other Party or its Affiliates in any press release, publication or other form of promotional disclosure without the prior written consent of the other Party in each instance. |
| 14.1.2 | Public Statements. Except as expressly set forth herein, each Party agrees not to issue any press release or other public statement or any information relating to this Agreement, whether written, electronic, oral or otherwise, disclosing the existence of this Agreement or the terms hereof or any other information relating to this Agreement without the prior written consent of the other Party; provided, however, that neither Party will be prevented from complying with any duty of disclosure it may have pursuant to Applicable Laws or the rules of any recognized stock exchange, including disclosure of the terms of this Agreement, so long as the disclosing Party provides the other Party at least ten (10) Business Days prior written notice to the extent practicable and only discloses information to the extent required by Applicable Law or the rules of any recognized stock exchange. Promptly after the Effective Date, the Parties will agree upon and release a mutual press release to announce the execution of this Agreement. |
| 14.1.3 | Publications. LICENSEE acknowledges that NOVARTIS personnel may desire to publish in scientific journals or present at scientific conferences scientific, pre-clinical or clinical data derived from research and development related to the Compounds and Products in the Field that was conducted by NOVARTIS or its Affiliates prior to the Effective Date. No such publication will be submitted and no such presentation shall be made unless a written copy of such proposed publication or presentation is submitted to LICENSEE no later than thirty (30) days before submission for publication or presentation. LICENSEE shall provide its comments with respect to such publications and presentations within fifteen (15) days after its receipt of such written copy from NOVARTIS. NOVARTIS shall consider in good faith all comments made by LICENSEE, including |
47
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| limitations on disclosure of NOVARTIS confidential information requested by LICENSEE consistent with what NOVARTIS would consider normal procedure for its own development compounds. LICENSEE and NOVARTIS will each comply with standard academic practice regarding authorship of scientific publications and recognition of contribution of other parties in any publication. |
| 15. | LICENSEE INSURANCE. LICENSEE shall maintain insurance during the Term, at its sole cost and expense, of the types and in amounts which are reasonable and customary in the U.S. pharmaceutical industry for companies of comparable size and activities obtained from a reputable insurer to protect against potential liabilities and risk arising out of activities to be performed under this Agreement and upon such terms (including coverages and deductible limits) as are customary in the U.S. pharmaceutical industry generally for the activities to be conducted by LICENSEE under this Agreement. Upon written request from NOVARTIS, LICENSEE shall promptly provide written evidence (e.g., certificates) of such insurance to NOVARTIS. |
| 16. | DISPUTE RESOLUTION |
| 16.1 | General. Promptly after the written request of either Party, each of the Parties shall appoint a designated representative to meet in person or by telephone to attempt in good faith to resolve any dispute that arises under this Agreement. If the designated representatives do not resolve the dispute within thirty (30) days of such request, then a senior executive of each Party shall meet in person or by telephone to review and attempt to resolve the dispute in good faith. The senior executives shall have thirty (30) days to attempt to resolve the dispute. If the senior executives cannot resolve such dispute within such period of time, then either Party shall have the right to institute binding arbitration as set forth in Section 16.2 upon written notice to the other Party. If a Party’s rights would be adversely affected as a result of the passage of time that would occur by participating in the dispute resolution mechanism set forth above, such Party may commence binding arbitration prior to or during the course of such dispute resolution mechanism. |
| 16.2 | Arbitration. Subject to Section 16.1, all controversies, disputes or claims arising out of or relating to this Agreement or any alleged breach hereof, will be settled exclusively by binding arbitration administered by the American Arbitration Association (“AAA”) pursuant to its Commercial Arbitration Rules then in effect, as supplemented by discovery pursuant to the Federal Rules of Civil Procedure. The place of arbitration shall be Boston, Massachusetts. The arbitration panel shall consist of a single neutral and independent arbitrator who is reasonably knowledgeable about the pharmaceutical industry and the subject matter at issue in the dispute (the “Arbitrator”). The Parties to the arbitration shall mutually agree on the Arbitrator. If the Parties cannot agree on an Arbitrator, the AAA shall select the Arbitrator, and the Arbitrator shall be and remain independent of the Parties. The Arbitrator shall determine what discovery will be permitted, consistent with the goal of reasonably controlling the cost and time that the |
48
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| Parties must expend for discovery; provided that the Arbitrator shall permit such discovery as he or she deems necessary to permit an equitable resolution of the dispute. The Parties shall use reasonable efforts to expedite the arbitration if requested by either Party. The Arbitrator shall, within fifteen (15) days after the conclusion of the arbitration hearing, issue a written award and statement of decision describing the essential findings and conclusions on which the award is based, including the calculation of any damages awarded. The award shall be final and binding on the Parties and non-appealable, and judgment upon the award rendered by the Arbitrator may be entered in any court of competent jurisdiction. The proceedings and the final award shall be confidential. All arbitration proceedings must be completed within one hundred eighty (180) days of the date of the notice instituting arbitration proceedings provided by a Party to the other Party pursuant to Section 16.1 or as soon as practicable thereafter. The question of arbitrability and whether a claim, dispute or other matter in question would be barred by the applicable statute of limitations, which statute of limitations also shall apply to any claim or dispute subject to arbitration under this Agreement, shall be determined by binding arbitration pursuant to this Section 16.2. Each Party shall bear its own fees costs and expenses (including attorneys’ fees and expenses), arising out of the arbitration described in this Section 16.2, and shall pay an equal share of the fees, costs and expenses of the Arbitrator and all other general fees related to the arbitration; provided, however, that the Arbitrator shall be authorized to allocate fees and expenses in a way that bears a reasonable relationship to the outcome of the arbitration, with the Party prevailing on more issues, or on issues of greater value or gravity, recovering a relatively larger share of its legal fees and expenses. Unless the Parties otherwise agree in writing, during the period of time that any arbitration proceeding is pending under this Agreement, the Parties shall continue to comply with all those terms and provisions of this Agreement that are not the subject of the pending arbitration proceeding. |
| 16.3 | Injunctive Relief. Notwithstanding the foregoing, in the event of an actual or threatened breach hereunder, the aggrieved Party may seek equitable relief (including restraining orders, specific performance or other injunctive relief) in any court or other forum, without first submitting to the dispute resolution procedures set forth in Sections 16.1 or 16.2. |
| 17. | GENERAL PROVISIONS |
| 17.1 | Assignment. LICENSEE may not assign its rights and obligations under this Agreement without NOVARTIS’ prior written consent, except that: (a) LICENSEE may assign its rights and obligations under this Agreement in whole or in part to one or more of its Affiliates without the consent of NOVARTIS; and (b) LICENSEE may assign this Agreement in the event of a Change in Control. LICENSEE shall provide NOVARTIS with prompt written notice of any such assignment. Any attempted assignment in contravention of the foregoing shall be void. NOVARTIS may assign its rights and obligations under this Agreement in whole or in part to one or more of its Affiliates without LICENSEE’s consent. |
49
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 17.2 | Severability. Should one or more of the provisions of this Agreement become void or unenforceable as a matter of law, then such provision will be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of this Agreement, and the Parties agree to substitute a valid and enforceable provision therefor which, as nearly as possible, achieves the desired economic effect and mutual understanding of the Parties under this Agreement. |
| 17.3 | Governing Law. This Agreement shall be governed by and construed under the laws in effect in the State of New York, without giving effect to any conflicts of laws provision thereof or of any other jurisdiction that would produce a contrary result. |
| 17.4 | Force Majeure. Any delay or nonperformance by such Party, for a period of up to 90 days, will not be considered a breach of this Agreement to the extent such delay or nonperformance is caused by acts of God, natural disasters, acts of the government or civil or military authority, fire, floods, epidemics, quarantine, energy crises, war or riots or other similar cause outside of the reasonable control of such Party (each, a “Force Majeure Event”), provided that the Party affected by such Force Majeure Event will promptly begin or resume performance as soon as reasonably practicable after the event has abated. |
| 17.5 | Waivers and Amendments. The failure of any Party to assert a right hereunder or to insist upon compliance with any term or condition of this Agreement shall not constitute a waiver of that right or excuse a similar subsequent failure to perform any such term or condition by the other Party. No waiver shall be effective unless it has been given in writing and signed by the Party giving such waiver. No provision of this Agreement may be amended or modified other than by a written document signed by authorized representatives of each Party. |
| 17.6 | Relationship of the Parties. Nothing contained in this Agreement shall be deemed to constitute a partnership, joint venture, or legal entity of any type between NOVARTIS and LICENSEE, or to constitute one Party as the agent of the other. Moreover, each Party agrees not to construe this Agreement, or any of the transactions contemplated hereby, as a partnership for any tax purposes. Each Party shall act solely as an independent contractor, and nothing in this Agreement shall be construed to give any Party the power or authority to act for, bind, or commit the other Party. |
| 17.7 | Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the Parties hereto and their respective successors and permitted assigns. |
| 17.8 | Notices. All notices, consents, waivers, and other communications under this Agreement must be in writing and will be deemed to have been duly given when: (a) delivered by hand (with written confirmation of receipt); or (b) when received |
50
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| by the addressee, if sent by an internationally recognized overnight delivery service (receipt requested), in each case to the appropriate addresses set forth below (or to such other addresses as a Party may designate by written notice): |
If to NOVARTIS:
Novartis International Pharmaceutical Ltd.
Xxxxxxxxxxxx 00
XX-0000 Xxxxx
Xxxxxxxxxxx
Attn: Head, NIBR Legal Europe
With a required copy to:
Novartis Institutes for BioMedical Research, Inc.
000 Xxxxxxxxxxxxx Xxxxxx
Xxxxxxxxx, XX 00000 XXX
Attn: General Counsel
If to LICENSEE:
Magenta Therapeutics, Inc.
00 Xxxxxxxxx Xxxxxx, 0xx Xxxxx
Xxxxxxxxx XX 00000
Attn: Xxxxxxxx Xxxxx and Xxxxxxxxx Xxxxxxx
With a copy to:
Ropes & Xxxx LLP
Attn: Xxxx X. Xxxxxxxxxx
Prudential Tower
000 Xxxxxxxx Xx.
Xxxxxx, XX 00000-0000
| 17.9 | Further Assurances. LICENSEE and NOVARTIS hereby covenant and agree without the necessity of any further consideration, to execute, acknowledge and deliver any and all such other documents and take any such other action as may be reasonably necessary or appropriate to carry out the intent and purposes of this Agreement. |
| 17.10 | No Third Party Beneficiary Rights. This Agreement is not intended to and shall not be construed to give any Third Party any interest or rights (including, without limitation, any third party beneficiary rights) with respect to or in connection with any agreement or provision contained herein or contemplated hereby. |
| 17.11 | Entire Agreement; Confidentiality Agreement. This Agreement, together with its Schedules, sets forth the entire agreement and understanding of the Parties as to the subject matter hereof and supersedes all proposals, oral or written, and all other prior communications between the Parties with respect to such subject |
51
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| matter, including, without limitation, that certain letter agreement by and between the Parties, dated November 16, 2016 (the “CDA”). The Parties acknowledge and agree that, as of the Effective Date, all Confidential Information (as defined in the CDA) disclosed by a Party or its Affiliates pursuant to the CDA shall be considered the Confidential Information of such Party and shall be subject to the terms set forth in this Agreement. In the event of any conflict between a material provision of this Agreement and any Schedule hereto, the Agreement shall control. |
| 17.12 | Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. An executed signature page of this Agreement delivered by facsimile transmission or other electronic format shall be as effective as an original executed signature page. |
| 17.13 | Cumulative Remedies. No remedy referred to in this Agreement is intended to be exclusive, but each shall be cumulative and in addition to any other remedy referred to in this Agreement or otherwise available under law. |
| 17.14 | Waiver of Rule of Construction. Each Party has had the opportunity to consult with counsel in connection with the review, drafting and negotiation of this Agreement. Accordingly, any rule of construction that any ambiguity in this Agreement shall be construed against the drafting Party shall not apply. |
| 17.15 | Construction. For purposes of this Agreement: (a) words in the singular shall be held to include the plural and vice versa as the context requires; (b) the words “including” and “include” shall mean “including, without limitation,” unless otherwise specified; (c) the terms “hereof,” “herein,” “herewith,” and “hereunder,” and words of similar import shall, unless otherwise stated, be construed to refer to this Agreement as a whole and not to any particular provision of this Agreement; (d) all references to “Section”, “Schedule” and “Exhibit,” unless otherwise specified, are intended to refer to a Section, Schedule or Exhibit of or to this Agreement; and (e) the term “or” shall be interpreted in the inclusive sense commonly associated with the term “and/or.” |
[Signatures on next page]
52
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
IN WITNESS WHEREOF, the Parties intending to be bound have caused this Agreement to be executed by their duly authorized representatives as of the Effective Date.
| NOVARTIS INTERNATIONAL PHARMACEUTICAL LTD. | MAGENTA THERAPEUTICS, INC. | |||||||
| By: | /s/ Lars Windhern | By: | /s/ Xxxxxxxx Xxxxx | |||||
| Name: | Lars Windhern | Name: | Xxxxxxxx Xxxxx | |||||
| Title: | Head Finance NIBR Europe | Title: | Chief Operating Officer | |||||
| By: | /s/ Simon Pfirter | |||||||
| Name: | Simon Pfirter | |||||||
| Title: | Authorized Signatory |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
Schedule 1.41
Knowledge of the Patent Associates
[***]
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
Schedule 1.74
Ultra Orphan Indications
[***]
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
SCHEDULE A
Licensed Patent Rights
[***]
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
SCHEDULE B-1
(a) Documents for Manufacturing of Product
| Media -fill protocols and reports | [***] | |
| Process B - BPRs | [***] | |
| Comparability report A’ vs B (fresh vs cryo) | [***] | |
| Cryo stability depleted fraction (incl comparability vials vs bags) | [***] | |
| Cryo stability expanded fraction (incl comparability vials vs bags) | [***] | |
| Site-to-site comparability (Apceth vs MCT) | [***] | |
| CMM stability report | [***] | |
| Compatibility report | [***] | |
| Engineering runs report process B at Apceth | [***] | |
| Day 0 report | [***] | |
| Analytical methods, protocols & reports | [***] | |
| Process B - release specifications | [***] | |
| List Raw Materials and Testing for Raw Materials | [***] | |
| List QC materials | [***] | |
| SFEM: pre-clinical report | [***] |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
(b) [***] Chemistry & Manufacturing Control Documents
| 1 | Drug Substance |
Certificate of Analysis
| • | For batch that will be transferred (No update according to latest specifications are planned) |
| 2 | Drug Product |
A. Manufacture
| • | Drug Product Manufacturing Instructions |
B. Drug Product Data
| • | Drug Product Specifications for early phase clinical development |
| • | Drug Product Analytical Methods |
| • | Drug Product Development Stability Report for development batches |
C. Certificate of Analysis
| • | For batches that will be transferred (No update according to latest specifications are planned) |
| 4 | Environmental Health and Safety |
| • | Safety Data Sheets of raw materials, intermediates, drug substance. |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
(c) Analytical Methods and Qualifications for HSC835
[***]
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
(d) Regulatory Documents
(Attached)
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
US Regulatory Record Indexing Report for INDs
| Application/ Registration Number | Product Code | Submission Date | Submission Folder Name | Content Summary | Submission Type Description | Serial/ Supplement Number | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] |
US Regulatory Record Indexing Report for ODAs
| Application/ Registration Number | Product Code | Submission Date | Submission Folder Name | Content Summary | Submission Type Description | Serial/ Supplement Number | ||||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||||
| [***] | [***] | [***] | [***] |
US Regulatory Record Indexing Report for INDs
| Application/ Registration Number | Product Code | Submission Date | Submission Folder Name | Content Summary | Submission Type Description | Serial/ Supplement Number | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
US Regulatory Record Indexing Report for INDs
| Application/ Registration Number | Product Code | Submission Date | Submission Folder Name | Content Summary | Submission Type Description | Serial/ Supplement Number | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
US Regulatory Record Indexing Report for INDs
| Application/ Registration Number | Product Code | Submission Date | Submission Folder Name | Content Summary | Submission Type Description | Serial/ Supplement Number | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | [***] | ||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | ||||||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] | [***] | [***] | [***] | [***] | ||||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] | [***] | [***] | |||||||
| [***] | [***] | [***] | [***] |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
(e) Market Analysis Material
(in PDF unless otherwise specified)
[***]
[***]
[***]
[***]
[***]
[***]
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
SCHEDULE B-2
Transfer Activities
| 1. | Regulatory Transfer Activities. The regulatory transfer activities shall be those activities set forth in this Section 1 of this Schedule. |
| 1.1. | Right of Reference of HemOnc IND: Within [***] days after the Effective Date, NOVARTIS shall provide to LICENSEE a copy of the HemOnc IND (in .pdf form) and will permit LICENSEE to reference the HemOnc IND pursuant to Section 4.3.1(a) of the Agreement, to enable LICENSEE to Develop and Commercialize Products in the Field that were submitted to the FDA by NOVARTIS prior to the Effective Date. At any time after LICENSEE’s receipt from NOVARTIS of a copy of the HemOnc IND, and a copy of the letter from Novartis to FDA granting the LICENSEE right of reference, the LICENSEE may provide to the applicable Regulatory Authority written notification (and any related necessary documents) of the right of reference of the HemOnc IND. |
| 1.2. | Assignment of IMD IND: |
| 1.2.1. | Within [***] days after written notification from LICENSEE that LICENSEE is able to assume all clinical, regulatory, and safety obligations (the “LICENSEE Assumption Notice”) regarding the IMD IND, and in no event longer than [***] days from the Effective Date, NOVARTIS shall execute and provide to LICENSEE documents (in a .pdf form) reasonably required to transfer the sponsorship of the IMD IND to LICENSEE. |
| 1.2.2. | Within [***] Business Days after LICENSEE’s receipt from NOVARTIS of the documents described in Section 1.2.1 of this Schedule, LICENSEE shall provide to the applicable Regulatory Authority written notification (and any related necessary documents) of the transfer of sponsorship of the IMD IND from NOVARTIS to LICENSEE (“LICENSEE Transfer Notice”). LICENSEE shall provide to NOVARTIS a copy of the LICENSEE Transfer Notice. In addition, LICENSEE shall provide to NOVARTIS a copy of any and all notices received by LICENSEE from the applicable Regulatory Authority confirming transfer of the IMD IND from NOVARTIS to LICENSEE (“Regulatory Confirmation Notice”). |
| 1.2.3. | For the period beginning on the Effective Date and ending on the effective date of the transfer to LICENSEE of the IMD IND (Le,, the date that LICENSEE serves official confirmation of acceptance of regulatory transfer of responsibility) (the “Regulatory Transfer Period”), NOVARTIS shall continue to maintain the IMD IND. For clarity, (a) during the Regulatory Transfer Period, NOVARTIS shall not be permitted to cancel or withdraw the IMD IND, and (b) NOVARTIS will file on a timely basis any annual report for the IMD IND due during the Regulatory Transfer Period. |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 2. | Documentation Transfer |
| 2.1. | Initial Request. No later than thirty (30) days after the Effective Date (unless otherwise specified within Schedule B-1 or agreed to in writing by the Parties), NOVARTIS will provide to LICENSEE, copies of those documents set forth in Section 2.3 of this Schedule (the “Documentation”). |
| 2.2. | Method of Transfer. Notwithstanding the foregoing, the Parties agree as follows with respect to the Documentation: NOVARTIS will provide electronic copies (in .pdf form unless otherwise specified) of the Documentation by a method reasonably acceptable to LICENSEE; provided that, to the extent such Documentation exists as of the Effective Date in an electronic format, NOVARTIS shall provide to LICENSEE an electronic copy of such Documentation and to the extent such Documentation does not exist in an electronic format as of the Effective Date, NOVARTIS shall provide to LICENSEE a physical copy of the Documentation. Notwithstanding the foregoing, in no event shall NOVARTIS be required to provide (i) data or records that include technology or products other than those that pertain to the Compounds, or (ii) laboratory notebooks, internal team meeting minutes, personal notes of NOVARTIS employees or any of NOVARTIS’ contractors or subcontractors, or internal intra-NOVARTIS correspondence. |
| 2.3. | Documentation. The Documentation shall be comprised of the following documents to the extent containing Licensed Know-How and Controlled by NOVARTIS or its Affiliates as of the Effective Date: |
| 2.3.1. | Regulatory and Manufacturing. As identified in Schedule B-1. |
| 2.3.2. | Market Analysis Materials. As identified in Schedule B-1. This section of Schedule B-1 is not subject to further revision pursuant to Section 3.2 of the Agreement. Materials will be provided “as is” with no warranty as to completeness or correctness and LICENSEE assumes full responsibility in using such Market Access Materials or any data or information contained therein. |
| 2.3.3. | Clinical Development. Copies of the following items that are Controlled by NOVARTIS, its Affiliates or the University of Minnesota (in the case of the University of Minnesota, in NOVARTIS’ possession) in each case below, solely as they relate to the HemOnc IND and the IMD IND: |
| 2.3.3.1. | An electronic copy of the then current databases as of the Effective Date, (a) the clinical study database, (b) the safety database, and (c) the PK and biomarker database. In addition, for study 2204, Novartis will refresh the safety database annually, or on an as requested basis, in no case more frequently than on a semi-annual basis; |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
| 2.3.3.2. | material protocols and amendments, study reports and results (including tables, figures and data); |
| 2.3.3.3. | All adverse event reports (e.g., Medwatch or equivalent forms); |
| 2.3.3.4. | Case Report Forms (CRFs) or equivalents thereof for all completed clinical studies of the Products (i.e., studies with signed-off final clinical study reports); |
| 2.3.3.5. | An electronic copy of clinical study raw data from clinical studies included in study databases; |
| 2.3.3.6. | all Trial Master Files (TMF’s) or equivalents thereof, to the extent such TMFs are in NOVARTIS’ or its Affiliates’ possession; and |
| 2.3.3.7. | To the extent any of the Documentation set forth in Sections 2.3.3.1—2.3.3.6 is in possession of the University of Minnesota as of the Effective Date, then Novartis agrees to notify the University of Minnesota of the license grant under this Agreement and will reasonably cooperate with LICENSEE to request and obtain such Documentation. |
| 2.3.4. | Intellectual Property. Copies of file wrappers for the Licensed Patent Rights in the Major Markets, will be delivered to LICENSEE within thirty (30) days of the Effective Date; records will be provided in .pdf form. |
| 3. | Access to Certain NOVARTIS Employees. For the period beginning on the Effective Date and ending [***] months after the Effective Date, NOVARTIS shall make the employees set forth on Schedule 3 available to assist LICENSEE or its designee from time to time as reasonably requested by LICENSEE (to the extent the same continue to work for NOVARTIS or its Affiliates) in order to provide LICENSEE with Information relating to the Development and manufacture of the Compounds and Products, [***]. |
| 4. | Conflicts. In the event of any conflict between this Schedule B-2 and the main text of the Agreement, the main text of the Agreement shall govern. |
CONFIDENTIAL TREATMENT REQUESTED. INFORMATION FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED IS OMITTED AND MARKED WITH “[***]”. AN UNREDACTED VERSION OF THE DOCUMENT HAS ALSO BEEN FURNISHED SEPARATELY TO THE SECURITIES AND EXCHANGE COMMISSION AS REQUIRED BY RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
Schedule 3
NOVARTIS Employees
[***]

