EXCLUSIVE LICENSE AGREEMENT
Exhibit 10.11
Pages where confidential treatment has been requested are stamped ‘Confidential Treatment Requested and the
Redacted Material has been separately filed with the Commission,’ and the confidential section has been
marked as follows: [***].
EXCLUSIVE LICENSE AGREEMENT
This Exclusive License Agreement (Agreement), effective as of this day of November 1, 2001
(the “Effective Date”), is by and between Cambridge University Technical Services Ltd., an English
limited company (“CUTS”) and Ceres, Inc. (“Ceres”), a Delaware corporation, having a principal
place of business at 0000 Xxxxxx Xxxxxx Xxxx, Xxxxxx, Xxxxxxxxxx 00000.
W I T N E S S E T H:
WHEREAS, The Chancellor, Masters and Scholars of the University of Cambridge and Ceres have
entered into that certain Sponsored Research Agreement dated June 1, 2000 in support of research
and development work, including the screening of certain transgenic plants;
WHEREAS, CUTS is the owner of certain patent rights and other intellectual property developed
by Xx. Xxxxxxxx relating to (i) Arabidopsis transgenic plants and (ii) the HAP-1 and other
extensin-GFP constructs and Ceres desires to license such rights from CUTS;
WHEREAS, CUTS desires to provide to Ceres certain first rights of refusal to Other Project
Technology (defined below); and
WHEREAS, Ceres desires to grant to the University of Cambridge license rights to certain of
Ceres’ technologies for the University’s non-commercial research and teaching activities.
NOW, THEREFORE, in consideration of the foregoing premises and the following mutual covenants,
and other good and valuable consideration, the receipt of which is hereby acknowledged, and
intending to be legally bound hereby, the Parties agree as follows:
1. DEFINITIONS
As used in this Agreement, the following terms will have the meaning set forth below:
1.1 “Affiliate(s)” shall mean (a) any company owned or controlled to the extent of at least
fifty percent (50%) of its issued and voting capital by a Party to this Agreement and any other
company so owned or controlled (directly or indirectly) by any such company or the owner of any
such company, or (b) any partnership, joint venture or other entity directly or indirectly
controlled by, controlling, or under common control with, to the extent of at least fifty percent
(50%) of voting power (or otherwise having power to control its general activities), a Party to
this Agreement.
1.2 “Background Technology” shall mean the Technologies developed prior to the Effective Date
of the Sponsored Research Agreement, which CUTS or Ceres owns, or has license rights to, and which
are useful for the Purpose. The Party’s respective Background Technology shall be referred to as
Ceres Background Technology and CUTS Background Technology. CUTS Background Technology shall
include certain CUTS Technology Rights relating to Arabidopsis transgenic plants, as listed in
Exhibit A; except that CUTS Background Technology shall not include any HAP-1 Technology, nor any
Project Technology. In addition, Ceres Background Technology shall include Technologies relating to
recombinant transmembrane proteins as defined in Exhibit I to Amendment I to the Sponsored Research
Agreement, developed prior to the Effective Date of such Amendment I.
1.3 “Biological Material” means any plants, seeds, microorganisms, cells, parts of cells, DNA,
RNA, cDNA, proteins, peptides, enzymes, and any combination of the foregoing, and/or other organic
matter and/or biologically active compounds.
1.4 “Biological Product(s)” means any product comprising the Biological Materials.
1.5 “Confidential Information” means any information, disclosed by one Party to this Agreement
to the other Party, that has any commercial value to the disclosing Party’s business, research,
development or other activities. Confidential Information includes, without limitation,
inventions, biological materials, technical information, trade secrets, financial information,
product plans, customer lists, marketing plans and strategies, forecasts and other business
information, improvements, ideas, works of authorship, processes, computer programs, techniques,
schematics, data, gene sequences, gene expression data, protein sequences, protein structures,
regulatory sequences, and other data.
1.6 “HAP-1 Patent” shall mean any Patent Rights based on the patent application listed in
Exhibit B, which includes the HAP-1 Technology.
1.7 “HAP-1 Products” shall mean all products, processes, or services including Biological
Products that are to be commercialized, the manufacture, use or sale of which is covered by any
valid and subsisting claim of the HAP-1 Patent.
1.8 “HAP-1 Technology” means any and all Technology related to and including (i) the HAP-1
sequences or constructs and (ii) the extensin GFP gene constructs, as further described in Exhibit
B.
1.9 “Net Sales” shall mean the gross amount actually received on sales of HAP-1 Products to
third parties (except as set forth below) by Ceres, and its Affiliates, less the following: (i)
customary trade, quantity, or cash discounts and commissions to non-affiliated brokers or agents to
the extent actually allowed and taken; (ii) amounts repaid or credited by reason of rejection or
return; (iii) any sales, use, tariff, customs duties, V.A.T. and/or other taxes, duties and similar
governmental assessments (except taxes based on income), which are paid by or on behalf of Ceres;
and (iv) outbound transportation, shipping, packing, costs of insurance in transit, and other costs
paid or allowed by Ceres; subject in all cases (i) to (iii) being separately
- 2 -
charged on customer invoices or credit notes. In any transfers of Product between Ceres and
an Affiliate, Net Sales shall be calculated based only on the final sale of the Product to an
independent third party.
1.10 “Non-Commercial Research and Teaching” shall mean research and teaching activities whose
primary purpose is the advancement of science or academic learning and dissemination of knowledge
excluding (a) any research sponsored by a commercial entity other than Ceres or (b) any research
where the results will be provided to a commercial entity other than Ceres, either directly by CUTS
or the University or via collaboration or otherwise, and other than through publication in a
learned journal.
1.11 “Other Project Technology” shall mean any Technology, which the University develops,
which is based on or created by using Project Technology and/or which furthers the Purpose without
funding or information from Ceres during the Research Project.
1.12 “Party” means, CUTS or Ceres, collectively they are sometimes referred to as the
“Parties”.
1.13 “Patent Rights” shall mean all patents and patent applications throughout the world,
including any reissues, extensions, substitutions, continuations, divisions, and
continuations-in-part applications, reexaminations or extensions or other government actions which
extend the life of a patent, and all rights to apply for patent protection and all rights, if any,
to xxx or bring other actions for past, present or future infringement of such rights. The Party’s
respective Patent Rights shall be referred to as Ceres Patent Rights and CUTS Patent Rights.
1.14 “Products” shall mean all products, processes, or services including Biological Products
that are to be commercialized, the manufacture, use or sale of which is covered by any valid and
subsisting claim of the Patent Rights within CUTS Background Technology, and/or the Project
Technology, except that Products shall not include any HAP-1 Products.
1.15 “Project Technology” shall mean any and all Technology developed or obtained during and
resulting from the Research Project, but excluding any Background Technology, HAP-1 Technology and
Other Project Technology.
1.16 “Purpose” shall mean the generation and analysis and transfer to Ceres of data and large
numbers of transgenic Arabidopsis plants and/or seeds transformed with constructs containing the
GFP gene the expression of which is indirectly driven by a large amount of different plant
promoters, as further described in the Research Plan.
1.17 “Research Project” shall mean the collaborative research program under which the
University and Ceres have performed and shall perform certain research and development activities
in pursuit of the Purpose and in accordance with the Research Plan, as defined in the Sponsored
Research Agreement.
- 3 -
1.18 “Research Plan” shall mean the mutually agreed document attached as Exhibit A to the
Sponsored Research Agreement that describes the respective research experiments and the specific
responsibilities of Ceres and University in performing the Research Project.
1.19 “Sublicense Income” shall mean the gross amount actually received by either Ceres or its
Affiliates in consideration for sublicenses of any of the rights under the HAP-1 Patent granted
hereunder, including up-front fees, lump sum payments and any running royalties (on a
product-by-product and country-by-country basis), without deduction of any kind, but excluding the
following, in relation to which no payments shall be due to CUTS:
(a) Payments received by either Ceres or its Affiliates solely for performance of research and
development, including but not limited to milestone payments for achievement of objectives in
research and development, only to the extent that such payments (i) cover the actual cost of the
research and development work; (ii) cover the amounts of the milestone payments due under Paragraph
5.5 hereof; or, (iii) are directly related to development of products that would be covered by this
Agreement;
(b) Investments made by a sublicensee in either Ceres or its Affiliates;
(c) Payments made to either Ceres or its Affiliates solely to the extent that they cover the
actual costs of conducting testing and other activities in connection with obtaining regulatory
approval for a Product;
(d) Reimbursed expenses of either Ceres or its Affiliates.
1.20 “Sponsored Research Agreement” shall mean that certain sponsored research agreement dated
June 1, 2000, between the University and Ceres in respect of the Research Project.
1.21 “Technological Element” shall mean any individual Biological Material, data, methods,
protocols, procedures, processes arising out of the University’s performance of the Research
Project and which is employed or embodied in a Product and which can be separated from other such
Biological Material, data, methods, protocols, procedures, processes employed or embodied in the
Product and which, in the absence of a license, would infringe CUTS Patent Rights. There shall be
three (3) mutually exclusive types of Technological Elements, (i) those solely owned by CUTS, (ii)
those jointly owned by CUTS and Ceres, and (iii) all other Technological Elements. A non-inclusive
list of examples of Technological Elements can be found in Exhibit C hereto.
1.22 “Technology” shall mean any Biological Material, Biological Product, data, methods,
protocols, procedures, processes and the like, and the Patent Rights and Technology Rights relating
thereto.
1.23 “Technology Rights” shall mean existing and future proprietary rights, including but not
limited to know-how rights, trade secret rights, copyrights, design rights, and all other
- 4 -
intellectual property rights (including without limitation the right, if any, to xxx or bring
other actions for past, present or future infringement of such proprietary rights), but excluding
Patent Rights. The Party’s respective Technology Rights shall be referred to as Ceres Technology
Rights and CUTS Technology Rights.
1.24 “Term” shall mean the period beginning on the Effective Date, and ending on the earlier
of (i) the date of the expiration of the last to expire patent licensed hereunder, or if no patents
are licensed hereunder, ten (10) years from the Effective Date, or (ii) the termination hereof
pursuant to the terms of Section 6 of this Agreement.
1.25 “University” shall mean the University of Cambridge.
2. PROJECT TECHNOLOGY
CUTS acknowledges and agrees with Section 4.2 of the Sponsored Research Agreement on Ownership
of Project Technology.
3. LICENSE GRANTS
3.1 Subject to all the terms of this Agreement, CUTS hereby grants to Ceres, under CUTS Patent
Rights and CUTS Technology Rights, a fully paid-up, irrevocable, world-wide, non-exclusive license,
including the right to grant sublicenses, to make, have made, use, or have used, CUTS Background
Technology.
3.2 Subject to all the terms of this Agreement Ceres hereby grants to CUTS a limited license
to use the Project Technology solely for Non-Commercial Research and Teaching purposes. Ceres
acknowledges and agrees that such license will be implemented by CUTS solely at the University,
under the full responsibility of CUTS.
3.3 Subject to all the terms of this Agreement CUTS hereby grants to Ceres, under CUTS Patent
Rights and CUTS Technology Rights an irrevocable, world-wide, exclusive license, including the
right to grant sublicenses: (a) to possess, to make (e.g. propagate), have made, use, sell, have
sold, offer for sale, import and have imported the HAP-1 Technology, and (b) to make, have made,
use, sell, have sold, offer for sale, import and have imported HAP-1 Products; except that CUTS
shall retain the limited right to use such HAP-1 Technology for Non-Commercial Research and
Teaching purposes solely at the University under the full responsibility of CUTS.
3.4 Ceres will notify CUTS of each sublicense granted hereunder.
3.5 Subject to all the terms of this Agreement, and only in respect of Ceres Background
Technology that Ceres makes available to the University for conducting the Research Project, Ceres
hereby grants to CUTS, under Ceres Patent Rights and Ceres Technology Rights, a limited, fully
paid-up, non-exclusive license to use such Ceres Background Technology solely at the University and
only (i) to perform the Research Project and (ii) for
- 5 -
Non-Commercial Research and Teaching purposes.
4. OTHER PROJECT TECHNOLOGY
Prior to commercializing or granting any rights to any third party to commercialize any Other
Project Technology, CUTS shall offer to Ceres in writing terms for the commercialization of such
Other Project Technology (“Offer”), and Ceres will have a sixty (60) day period from the date of
delivery of the Offer in which to indicate its desire to accept such Offer, subject to negotiation
of definitive agreements. If the Offer is declined or is not accepted during such sixty (60) day
period, CUTS may either commercialize itself or grant a license to a third party on terms no more
favorable to the third party than the terms of the Offer. If Ceres indicates its desire to accept
the Offer in writing within said sixty (60) day period, the Parties agree to negotiate in good
faith the definitive agreements for such commercialization; provided, however, that if the Parties
are unable to agree upon the terms and conditions of any such license or acquisition agreement
within twelve months of delivery of the written notice of Ceres’ desire to accept the Offer, CUTS
may either commercialize itself or grant a license to a third party on terms no more favorable to
the third party than the terms offered by CUTS to Ceres. In determining whether the terms offered
to a third party are more favorable than those received or accepted by Ceres, all terms and
conditions of the respective offers shall be considered, including but not limited to, monetary
terms, scope of rights granted, warranties and indemnities.
5. CONSIDERATION
5.1 License Initiation Fee. Ceres agrees to pay to CUTS the amount of [***] United
States dollars (U.S. $[***]) within thirty (30) business days of the Effective Date. Such fee
includes the reimbursement of all costs incurred by CUTS in connection with the filing and
prosecution of the HAP-1 Patent.
5.2 Royalty on Net Sales. Ceres shall pay to CUTS an earned royalty on Net Sales on a
product-by-product and country-by-country basis. Earned royalties shall accrue in each country for
the duration of CUTS Patent Rights covering such HAP-1 Product in that country. Ceres shall pay
royalties to CUTS on Net Sales from the first date of commercial introduction of a HAP-1 Product,
which royalty shall be A. A is defined as follows: the lesser of [***] percent ([***]%) or
(W/X)(MRNS); where X shall be the total number of Technological Elements in the HAP-1 Product; W is
the sum of one half of the number of Technological Elements contributed to the HAP-1 Product which
are jointly owned by CUTS and Ceres plus the total number of Technological Elements contributed to
the HAP-1 Product solely by CUTS; and, MRNS shall be the maximum royalty on Net Sales, which is
[***] percent ([***]%). The Parties further agree to negotiate in good faith for another royalty
rate in the event of substantial market considerations.
5.3 Royalty on Sublicense Income. Ceres shall pay to CUTS an earned royalty on
Sublicense Income actually received by Ceres, which royalty shall be A. A is defined as follows:
the lesser of [***] percent ([***]%) or (W/X)(MRNS); where X shall be the total number of
Technological Elements in the sublicense; W is the sum of one half of the number of Technological
Elements contributed to the sublicense which are jointly owned by CUTS and
- 6 -
| Confidential Treatment Requested and the Redacted Material has been separately filed with the Commission. | ||
Ceres plus the total number of Technological Elements contributed to the sublicense solely by
CUTS; and, MRNS shall be the maximum royalty on Sublicense Income, which is [***] percent ([***]%).
The Parties further agree to negotiate in good faith for a lower royalty rate in the event of
substantial market considerations.
5.4 Milestone Payments. Ceres shall pay to CUTS the following Milestone payments:
(a) within thirty (30) days of Ceres’ successful validation of the technical evaluation
protocol for a dicotyledon plant, such as Arabidopsis, associated with the HAP-1 Technology and
described in Exhibit B of this Agreement, Ceres shall pay to CUTS [***] United States dollars (U.S.
$[***]). Ceres shall use all reasonable efforts to complete such validation within two (2) years
of the Effective Date;
(b) within thirty (30) days of Ceres’ successful validation of the technical evaluation
protocol for a monocotyledon plant, such as rice, associated with the HAP-1 Technology and
described in Exhibit B of this Agreement, Ceres shall pay to CUTS [***] United States dollars (U.S.
$[***]);
(c) within thirty (30) days of CUTS’ notice to Ceres that a United States patent under the
HAP-1 Patent, licensed to Ceres hereunder has been issued to CUTS, Ceres shall pay to CUTS [***]
United States dollars (U.S. $[***]);
(d) within thirty (30) days of CUTS’ notice to Ceres that a European patent under the HAP-1
Patent licensed to Ceres hereunder, has been issued to CUTS, Ceres shall pay to CUTS [***] United
States dollars (U.S. $[***]).
5.5 Royalty Reports; Payments; Records
(a) First Sale. Ceres shall report to CUTS the date of first commercial sale of any HAP-1
Product within thirty (30) business days of occurrence in each country.
(b) Reports and Payments. Within sixty (60) days after the conclusion of each calendar year
following First Sale, Ceres shall deliver to CUTS a report containing the following information:
- 7 -
| Confidential Treatment Requested and the Redacted Material has been separately filed with the Commission. | ||
(i) the number of HAP-1 Products sold to independent third parties in each
country;
(ii) the gross sales price for each HAP-1 Product sold by Ceres and its
Affiliates during the applicable year in each country;
(iii) the calculation of Net Sales and Sublicense Income for the applicable
year in each country, including a listing of applicable deductions and
credits applied;
(iv) the total royalty payable on Net Sales in U.S. dollars, together with
the exchange rates used for conversion; and,
(v) a statement indicating whether any Milestones have been attained
pursuant to Section 5.5.
All such reports shall be considered Ceres Confidential Information pursuant to Section 7, and
shall not be transferred to a third party. If no royalties are due to CUTS for any year, the
report shall so state. Concurrent with this report, Ceres shall remit to CUTS any payment due in
respect of Net Sales, Sublicense Income or Milestones for the applicable year. CUTS shall instruct
Ceres as to the method of payment.
(c) Records. Ceres shall maintain, and shall cause its Affiliates to maintain, reasonably
complete and accurate records of HAP-1 Product that is made, used, sold, or performed and
Sublicense Income received under this Agreement and any amounts payable to CUTS in relation to such
HAP-1 Product, which records shall contain sufficient information to permit CUTS to confirm the
accuracy of any reports delivered to CUTS under this Section 5.6. The relevant party shall retain
such records relating to a given royalty period for at least three (3) years after the conclusion
of that royalty period, during which time CUTS shall have the right, at its sole expense, to cause
an independent, certified public accountant to inspect such records once per calendar year, upon
thirty (30) days’ prior written notice, during normal business hours for the sole purpose of
verifying any reports and payments delivered under this Agreement. The parties shall reconcile any
underpayment or overpayment within thirty (30) days after the accountant delivers the results of
the audit. In the event that any audit performed under this Section reveals an aggregate
underpayment in excess of five percent (5%) during any calendar year, Ceres or the applicable
sublicensee or Affiliate shall bear the full cost of such audit.
6. TERM AND TERMINATION
6.1 Term. Unless otherwise terminated by operation of law or by acts of the Parties
in accordance with the terms of this Agreement, this Agreement will be in force for the Term.
6.2 Monies Due/Accrued Rights. Any termination of this Agreement shall not relieve
Ceres of its obligation to pay any monies due or owing at the time of such termination and will not
impair any accrued right of CUTS arising under this Agreement prior to such termination.
- 8 -
6.3 Termination Upon Breach. Upon material breach or default of any of the terms and
conditions of this Agreement, the defaulting Party shall be given notice of such default in writing
and a period of sixty (60) days after receipt of such notice to correct the breach or default. If
(a) the default or breach (i) is material to this Agreement taken as a whole, and (ii) is not
corrected within said sixty (60) day period and the defaulting Party has not taken reasonable steps
to cure the same, and (b) the Party not in default has fully complied with all of its obligations
under this Agreement and (c) the Party not in default has no adequate remedy from monetary changes,
the Party not in default shall have the right to terminate this Agreement. In the event that this
Agreement is terminated due to a breach by CUTS, Sections 3.1 and 3.3 of this Agreement shall
survive such termination.
6.4 Termination upon Bankruptcy. A Party shall have the right to terminate this
agreement upon the first to occur of the following events: (i) a petition of action is filed or
action taken by or against the other Party under any law dealing with insolvency or bankruptcy;
(ii) a receiver is appointed over the assets or undertaking of the other Party; (iii) the other
Party enters into a deed of arrangement or makes an assignment for the benefit of creditors; or
(iv) the other Party ceases to function as a going concern or an order is made or a resolution
passed to that effect.
6.5 Ceres Termination Rights. In addition to the above termination rights, Ceres
shall be entitled to terminate this Agreement at any time, with or without cause, upon providing
CUTS with ninety (90) days’ notice of termination in writing.
7. CONFIDENTIALITY
7.1 Mutual Non-Disclosure Obligations. Without prejudice to Sections 7.7 and 7.8 of
the Sponsored Research Agreement, each Party hereby agrees that it shall keep confidential and not
use for any purpose, except as provided herein, all Confidential Information supplied to it (the
“Recipient”) by the other Party (the “Disclosing Party”) during the term of this Agreement and for
five (5) years after termination or expiration hereof; provided, however, that the foregoing
obligations of confidentiality and non-use shall not apply to the extent that any Confidential
Information is demonstrated by written records to be (a) already known to the Recipient or one of
its Affiliates at the time of disclosure hereunder (provided the Recipient and/or its Affiliates
comply with any restrictions imposed by third parties); or (b) is hereafter developed by the
Recipient or one of its Affiliates in the course of work entirely independent of any disclosure
hereunder; or (c) publicly known prior to or after disclosure hereunder other than through acts or
omissions of the Recipient or one of its Affiliates; or (d) disclosed in good faith to the
Recipient or one of its Affiliates by a third party (provided the Recipient and/or its Affiliates
comply with any restrictions imposed by third parties). This does not prevent disclosure to third
parties by the Recipient under a secrecy or confidentiality agreement with essentially the same
confidentiality provisions provided herein in connection with the exercise of its rights under this
Agreement (but only to the extent permitted herein). In addition, disclosure may be made (i) to
Recipient’s employees, consultants, representatives, agents and advisors provided that such persons
are subject to confidentiality obligations consistent with the ones set
- 9 -
forth in this Section 7.1, and (ii) to governmental agencies to the extent required to secure
governmental approval for marketing of the Products; provided, however, that the Recipient shall
seek to limit disclosure and to obtain confidential treatment therefor.
7.2 Affiliates, Licensee and Sublicensees. Nothing herein shall be construed as
preventing Ceres from disclosing any information received from CUTS to an Affiliate, licensee or to
a sublicensee of Ceres, provided such Affiliate, licensee or sublicensee has undertaken a similar
obligation of confidentiality with respect to the Confidential Information.
7.3 Internet Communications. To the extent that the Parties use the Internet as a
means of communication, all e-mail and/or other Internet-based communications containing
Confidential Information shall be encrypted.
8. PRESS RELEASES AND USE OF NAMES AND TRADEMARKS
8.1 Press Releases. All press releases which one Party desires to make relating to
the Research Project or any of the matters contemplated hereunder shall be prepared by such Party
as a joint press release of the Parties and shall not be publicly released or released to the press
without the prior written consent of the other Party.
8.2 Use of Tradenames. Neither Party shall disclose or use the name of the other for
any purpose without the prior written consent of the named Party, except for the purposes of
referring to this Agreement in disclosures to be made in documents in connection with financings
and/or as required by law.
9. REPRESENTATIONS AND WARRANTIES
9.1 Ceres Representations and Warranties. Ceres represents to CUTS that:
(a) Ceres is a corporation duly organized, validly existing and in good standing under the
laws of the State of Delaware and has all requisite corporate power and authority to carry on its
business as now conducted;
(b) All corporate action on the part of Ceres and its officers and directors necessary for the
authorization, execution and delivery of this Agreement and the performance of all obligations of
Ceres hereunder has been taken, and this Agreement constitutes a valid and legally binding
obligation of Ceres, enforceable in accordance with its terms; and,
(c) Ceres warrants to CUTS that it has the lawful right to grant the licenses granted to CUTS
under this Agreement.
9.2 CUTS Representations and Warranties.
(a) CUTS is a company duly organized, validly existing and in good standing under the laws of
England and Wales has all requisite power and authority to carry on its
- 10 -
business as now conducted;
(b) All action on the part of CUTS and its officers and directors necessary for the
authorization, execution and delivery of this Agreement and the performance of all obligations of
CUTS hereunder has been taken, and this Agreement constitutes a valid and legally binding
obligation of CUTS, enforceable in accordance with its terms; and
(c) CUTS warrants to Ceres that it has the lawful right to grant the licenses granted to Ceres
under this Agreement. CUTS warrants that, to the best of its knowledge, CUTS owns and has full
rights, title and interest, through assignment by the University and/or inventors associated with
the University, in all Technology Rights, Patent Rights or other rights which are or shall or may
be licensed to Ceres pursuant to this Agreement; including without limitation, Technology Rights,
Patent Rights and other rights on CUTS Background Technology, on the HAP-1 Patent and HAP-1
Technology, and on Other Project Technology.
10. DISCLAIMERS
10.1 Project Technology and CUTS Background Technology. The Parties accept no
responsibility whatsoever for any use which may be made of any work carried out under or pursuant
to this Agreement, of the Project Technology, or of its Background Technology, and no liability
whatsoever either direct or indirect shall rest upon the a Party, its employees, students, agents
or appointees for the effects of any Product or process that may be developed, manufactured, used,
sold, imported or distributed by or on behalf of the other Party or any Affiliate or sublicensee of
the other Party, notwithstanding that such Product or process may be based upon the findings of the
Research Project, the results or upon any other advice or information furnished by a Party, its
employees, students, agents or appointees under this Agreement.
10.2 General. Except as expressly provided for in this Agreement the licenses granted
to the Parties under this Agreement and the associated Technology, Biological Materials, Patent
Rights, property rights, Products, and patent methods are provided WITHOUT WARRANTY OF
MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER REPRESENTATION OR WARRANTY,
EXPRESS OR IMPLIED.
11. LIMITATION OF LIABILITY
11.1 EXCLUSIONS. IN NO EVENT WILL ANY PARTY BE LIABLE UNDER ANY CONTRACT, NEGLIGENCE,
STRICT LIABILITY OR OTHER THEORY FOR ANY INDIRECT, INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES
INCLUDING, BUT NOT LIMITED TO, LOSS OF REVENUES AND LOSS OF PROFITS IN CONNECTION WITH THIS
AGREEMENT OR IN CONNECTION WITH THE USE OF THE TECHNOLOGY, BIOLOGICAL MATERIALS, PATENT RIGHTS,
PROPERTY RIGHTS OR PRODUCTS.
11.2 Limitation. Under no circumstances whatsoever shall CUTS liability to Ceres
- 11 -
under or otherwise in connection with this Agreement exceed sums paid by Ceres to CUTS under
this Agreement together with the sums paid by Ceres to the University under the Sponsored Research
Agreement.
12. INDEMNIFICATION
12.1 Ceres Indemnity. Ceres agrees to indemnify, defend and hold harmless CUTS, its
employees, students, agents and appointees, including but not limited to, Xx. Xxxxxxxx, from and
against any and all liability, loss, damage, cost or expense (including reasonable legal fees,
court costs and other expenses of litigation) arising out of or in connection with third party
claims relating to:
(a) any alleged infringement of a third party’s intellectual property rights by reason of
Ceres’ activities in relation to the Research Project or this Agreement; or
(b) any HAP-1 Product or process developed, manufactured, used, sold, imported or distributed
by or on behalf of Ceres, its Affiliates or sublicensees arising out of the Research Project or in
any way out of this Agreement.
13. PATENT PROSECUTION AND MAINTENANCE
13.1 Responsibility.
(a) CUTS will diligently prosecute and maintain at its own expense, CUTS Patent Rights related
to CUTS Background Technology and Other Project Technology using counsel of its choice.
(b) Ceres shall have the sole right and discretion, at its own expense, to prepare, file,
prosecute and maintain patent applications and patents claiming Project Technology and the HAP-1
Patent, using patent counsel of its own choosing. CUTS will cause inventors to assign their
ownership rights in the Project Technology to Ceres and will cooperate with and assist Ceres in
preparation of such patents and patent applications. CUTS will cooperate with and assist Ceres, and
will cause the University to cooperate with and assist Ceres in assuming any ongoing patent
prosecution relating to the HAP-1 Patent or in the preparation and prosecution of any further such
patents and patent applications as may arise.
(c) The Parties will promptly provide each other with copies of all relevant documentation
associated with their respective Patent Rights to the extent that such Patent Rights relate to
Project Technology, the HAP-1 Patent, CUTS Background Technology or Other Project Technology and
all other reasonable assistance so that both Parties may be currently and promptly informed and
apprised of the continuing prosecution and may comment upon such documentation sufficiently in
advance of any initial deadline for filing a response; provided, however, that if the receiving
Party has not commented upon such documentation prior to ten (10) business days before the initial
deadline for filing a response with the relevant government patent office, then the Party providing
the documentation will be free to respond appropriately
- 12 -
without consideration of comments by the receiving Party, if any. Both Parties hereto will
keep this documentation in confidence in accordance with the provisions of Section 7 herein.
13.2 Choice to Not Prosecute. If subsequent to filing a patent application that
claims Project Technology or the HAP-1 Patent, Ceres elects not to prosecute or maintain such
patent application or ensuing patent or fund such prosecution, filing or maintenance, Ceres shall,
on a country-by-country basis, give CUTS notice thereof within a reasonable period prior to
allowing such patent application or patent to lapse or become abandoned or unenforceable and CUTS
may continue prosecution or maintenance of such patent application or patent at its sole expense
and for its exclusive benefit.
13.3 Claims. CUTS will use all reasonable efforts to amend any patent application to
include claims requested by Ceres and required to protect the products contemplated to be sold or
methods contemplated to be practiced under this Agreement.
13.4 Interferences/Oppositions. The costs of all interferences and oppositions
relating to such Patent Rights will be considered prosecution expenses and also will be borne by
the prosecuting Party.
14. PATENT INFRINGEMENT
14.1 Notice. In the event that a Party learns of any infringement of any Patent Right
licensed under this Agreement, that Party will call the attention of the other Party thereto in
writing and will provide the other Party with reasonable evidence of such infringement. The
Parties to this Agreement acknowledge and agree that during the period and in a jurisdiction where
Ceres has exclusive rights under this Agreement, CUTS will not notify a third party of the
infringement of any of Patent Rights without first obtaining consent of Ceres. The Parties will
use their diligent efforts in cooperation with each other to terminate such infringement without
litigation. Ceres shall have no obligation and CUTS shall have no right, to grant any rights to
such infringing third party in derogation of the exclusive licenses granted to Ceres under this
Agreement.
14.2 Legal Action. Ceres may request that CUTS take legal action against the
infringement of Patent Rights. Such request must be made in writing and must include reasonable
evidence of such infringement and damages to Ceres. If the infringing activity has not been abated
within ninety (90) days following the effective date of such request, CUTS will have the right to
elect to:
(a) commence suit on its own account; or
(b) refuse to participate in such suit;
and CUTS will give notice of its election in writing to Ceres by the end of the 100th day after
receiving notice of such request from Ceres. Ceres may thereafter bring suit for patent
infringement if and only if CUTS elects not to commence suit and if the infringement occurred
- 13 -
during the period and in a jurisdiction where Ceres had exclusive rights under this Agreement.
However, in the event Ceres elects to bring suit in accordance with this Section 14.2, CUTS may
thereafter join such suit at its own expense.
14.3 Expenses and Awards. Such legal action as is decided upon will be at the expense
of the Party on account of whom suit is brought and all recoveries recovered thereby will belong to
such Party; provided, however, that legal action brought jointly by CUTS and Ceres and participated
in by both will be at the joint expense of the Parties and all recoveries from such joint legal
action will be allocated in the following order:
(a) to each Party as reimbursement in equal amounts of the outside attorney’s costs, fees, and
other related expenses to the extent each Party paid for such costs, fees, and expenses until all
such costs, fees, and expenses are consumed for each Party, provided that if one Party paid more
for such costs, fees, and expenses, all of such Party’s costs, fees, and expenses shall be
reimbursed in full prior to the allocation of any remaining recovery to either Party under
Subsection 14.3(b); and
(b) any remaining amount to be divided by the Parties in the following manner: (i)
seventy-five percent (75%) to Ceres and twenty-five percent (25%) to CUTS of any recoveries based
on actual damages and lost profits; and (ii) fifty percent (50%) to Ceres and fifty percent (50%)
to CUTS of any recoveries based on punitive and statutory enhanced damages.
14.4 Cooperation. Each Party will cooperate with the other in litigation proceedings
instituted hereunder but at the expense of the Party on account of whom suit is brought. Such
litigation will be controlled by the Party bringing the suit, except that CUTS may be represented
by counsel of its choice, at its expense, in any suit brought by Ceres.
15. PATENT MARKING
Ceres will xxxx all Products made, used, or sold under the terms of this Agreement, or their
containers, in accordance with the applicable patent marking laws.
16. GOVERNMENT APPROVAL OR REGISTRATION
If this Agreement or any associated transaction is required by the law of any nation to be
either approved or registered with any governmental agency, Ceres will assume all legal obligations
to do so. Ceres will notify CUTS if it becomes aware that this Agreement is subject to a United
States or foreign government reporting or approval requirement. Ceres will make or cause to be
made all necessary filings and pay all costs including fees, penalties, and all other out-of-pocket
costs associated with such reporting or approval process. CUTS shall cooperate with Ceres, to the
extent it is able to do so within the law and established policy of CUTS, by providing
documentation and testimony to assist Ceres in obtaining such approval or registration. Any
expenses incurred by CUTS in cooperating with Ceres in obtaining approval or registration of this
Agreement in any country will be reimbursed within thirty (30) days after
- 14 -
receiving an itemized invoice for such expenses from CUTS.
17. MISCELLANEOUS
17.1 Reserved Rights. Ceres may during or after the Term of this Agreement
independently or with third parties perform any research, including, without limitation, research
related to the Purpose in any plant. Cambridge retains the right, after the Research Project is
complete, to perform research and development work related to the Purpose independent of Ceres.
17.2 Mediation and Governing Law.
(a) If any dispute arises out of or in connection with this Agreement the Parties will attempt
in good faith to settle it by negotiation.
(b) If the Parties are unable to settle any dispute by negotiation within twenty-eight (28)
days the Parties will attempt to settle it by mediation in accordance with the Centre for Dispute
Resolution (CEDR) Model Mediation Procedure.
(c) To initiate a mediation, a Party must give notice in writing to the other Parties
requesting a mediation in accordance with Section 17.7.
(d) This Agreement and all questions of construction, validity and performance under this
Agreement shall be governed by English law.
17.3 Independent Contractors. Nothing in this Agreement is intended or shall be
deemed to constitute a partnership, agency, employer-employee or joint venture relationship between
the Parties. All activities by the Parties hereunder shall be provided as independent contractors.
Neither Party shall incur any debts or make any commitments for the other except to the extent, if
at all, specifically provided herein.
17.4 Force Majeure. If the performance of any part of this Agreement by a Party is
prevented, restricted, interfered with or delayed by reason of any cause beyond the reasonable
control of the Party liable to perform, unless conclusive evidence to the contrary is provided, the
Party so affected shall use its diligent efforts to avoid or remove such causes of non-performance
and shall continue performance with the utmost dispatch whenever such causes are removed. When
such circumstances arise, the Parties shall discuss what, if any, modification of the terms of this
Agreement may be required in order to arrive at an equitable solution.
17.5 Construction. In the event any portion of this Agreement shall be held illegal,
void or ineffective, the remaining portions hereof shall remain in full force and effect, as long
as it does not materially alter the purpose and performance of this Agreement. If any of the terms
or provisions of this Agreement are in conflict with any applicable statute or rule of law, then
such terms or provisions shall be deemed inoperative to the extent that they may conflict therewith
and shall be deemed to be modified to conform with such statute or rule of law. In the
- 15 -
event that the terms and conditions of this Agreement are materially altered as a result of
this Section, the Parties will renegotiate the terms and conditions of this Agreement to resolve
any inequities.
17.6 Entire Agreement. Without prejudice to the Sponsored Research Agreement, this
Agreement constitutes the entire agreement between the Parties relating to the subject matter
hereof and supersedes all prior agreements, understandings, writings, and discussions between the
Parties relating to said subject matter. No terms or provisions of this Agreement shall be varied
or modified by any prior or subsequent statement, conduct or act of the Parties, except that the
Parties may amend this Agreement by written instruments specifically referring to and executed in
the same manner as this Agreement.
17.7 Notices. All notices pertaining to this Agreement, including but not limited to
notices concerning progress reports and royalty and other payments, shall be in writing and sent by
two-day delivery via an internationally recognized delivery service, to the Parties at the
following addresses or such other address as such Party shall have furnished in writing to the
other Parties in accordance with this Section 17.7:
For CUTS:
Cambridge University Technical Services, Ltd.
Research Services Division
00 Xxxx Xxxx
Xxxxxxxxx XX0 0XX
Great Britain
Attention: Contracts Officer
Research Services Division
00 Xxxx Xxxx
Xxxxxxxxx XX0 0XX
Great Britain
Attention: Contracts Officer
For Ceres:
A notice shall be deemed to have been received on the day after deposit with the delivery
service, if sent by overnight delivery.
17.8 No Third Party Beneficiaries and No Assignment. This Agreement shall be binding
upon and inure to the benefit of the successors in interest of the respective Parties. This
Agreement shall not be assigned by any Party without the written consent of the other Parties;
provided, however, Ceres may assign this Agreement to any Affiliates or to any corporation with
which it may merge or consolidate, or to which it may transfer all or substantially all of its
assets or business, without obtaining the consent of CUTS.
- 16 -
17.9 Further Assurances. Each Party hereto shall execute such further papers or
agreements as may be necessary to effect the purposes of this Agreement and carry out its
provisions.
17.10 Export Control Laws. The Parties will observe all applicable national laws with
respect to the transfer of materials and related technical data to foreign countries, including,
without limitation, the International Traffic in Arms Regulations (ITAR) and the Export
Administration Regulations.
17.11 No Waiver. The failure of either Party at any time or times to require
performance of any provision hereof shall in no manner affect its right at a later time to enforce
the same. No waiver by either Party of any condition or term in any one or more instances shall be
construed as a further or continuing waiver of such condition or term or of any other condition or
term.
17.12 Severability. If any term of this Agreement is held by a court of competent jurisdiction
to be unenforceable because it is invalid or in conflict with any law of any relevant jurisdiction,
the validity of the remaining provisions shall not be affected.
17.13
Headings. The headings of the several sections are inserted for convenience of
reference only and are not intended to be a part of or to affect the meaning or interpretation of
this Agreement.
17.14 Survival. Upon termination of this Agreement for any reason, Sections 3, 6, 7,
8, 10, 11, 12, 13, 14 and 17.2 and any accrued rights, obligations and causes of action shall
survive termination of this Agreement.
- 17 -
In Witness Whereof, both CUTS and Ceres have executed this Exclusive License Agreement, in
duplicate originals, by their respective officers hereunto duly authorized, as of the date and year
written on page one hereof.
| CAMBRIDGE UNIVERSITY TECHNICAL SERVICES LTD. | CERES, INC. | |||||
By
|
/s/ X.X. Xxxxxxxx | By | /s/ Xxxxxxx Xxxxxxx | |||
| Name: | Xx. X.X. Xxxxxxxx | Name: Xxxxxxx Xxxxxxx, CBE, FRS | ||||
| Title: | Director | Title: Chief Scientific Officer | ||||
| By | /s/ Xxxxx Xxxxxx | |||||
| Name: Xx. Xxxxx Xxxxxx | ||||||
| Title: Director of Product Development | ||||||
- 18 -
Exhibit A
CUTS Background Technology
CUTS Background Technology
HAP1 ENHANCER TRAP LINES
HAP1-VP16:HAP1-UAS-Extensin-emdGFP
HC10 — stomatal guard cells
HC03 — root base, cortex, endodermis
HC128.3 — petal vasculature
HC104.2 — leaf vasculature
HC03 — root base, cortex, endodermis
HC128.3 — petal vasculature
HC104.2 — leaf vasculature
HAP1-VP16:HAP1-UAS-histone-mCFP
With expression patterns in first screen:
HS69 — epidermis of roots, pili, abscission zones
HS135 — mature vasculature of silique and petals
HS151 — base of silique
HS164 — epidermis of roots, cotyledons, siliques, flowers
HS165 — style epidermis
HS176 — mature leaves
HS181 — silique vascular
HS135 — mature vasculature of silique and petals
HS151 — base of silique
HS164 — epidermis of roots, cotyledons, siliques, flowers
HS165 — style epidermis
HS176 — mature leaves
HS181 — silique vascular
HS222 — YOUNG ROOTS
HS230 — sepals and leaves
HS238 — vascular tissue roots, shoot meristem of embryo
HS241a — root tips
HS241b — hypocotyls and cotyledons, stem
HS251 — epidermis of stem/flower
HS357 — lateral root primordial
HS238 — vascular tissue roots, shoot meristem of embryo
HS241a — root tips
HS241b — hypocotyls and cotyledons, stem
HS251 — epidermis of stem/flower
HS357 — lateral root primordial
No expression in untreated seedlings, mature plants:
HS42
HS57
HS62
HS57
HS62
HS130
HS138
HS168
HS179
HS181
HS186
HS192
HS202
HS204
HS210
HS216
HS217
Hs220
HS221
HS225
HS228
HS231
HS232
HS243
HS247
HS250
HS253
HS359
HS630
HS361
HS364
HS366
HS368
HS369
HS371
HS138
HS168
HS179
HS181
HS186
HS192
HS202
HS204
HS210
HS216
HS217
Hs220
HS221
HS225
HS228
HS231
HS232
HS243
HS247
HS250
HS253
HS359
HS630
HS361
HS364
HS366
HS368
HS369
HS371
Exhibit B
HAP-1 Technology
HAP-1 Technology
Description of HAP-1 technology:
ET HAP1 EXT emdGFP [diagram of linear T-DNA vector transformation]
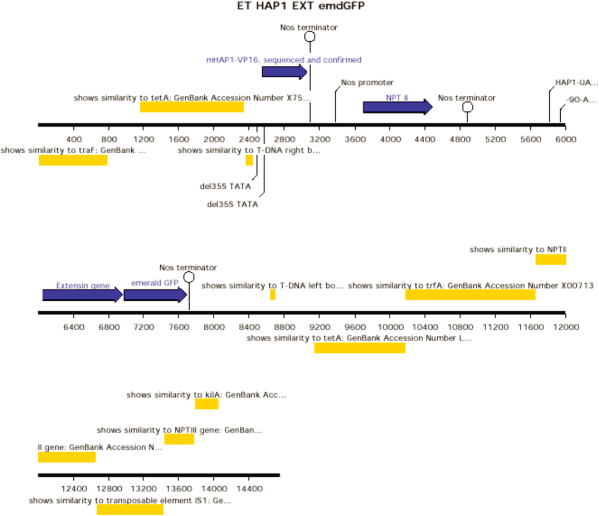




















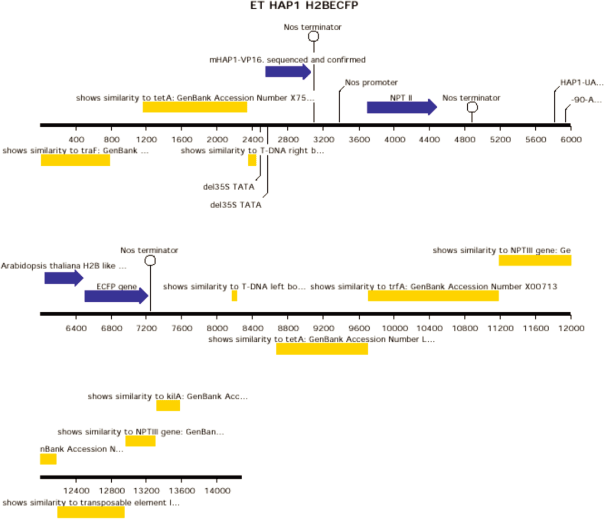
ET HAP1 H2BECFP [diagram of linear T-DNA vector transformation]

















HAP-1 Patent:
UK priority patent application number 0122828.7 filed on September 21, 2001, with title: Gene
expression construct.
Protocol associated with the validation of the use of HAP1 within the HAP-1 Technology:
Transgene activation by HAP1 in dicots: the following milestones should be achieved by
December 2002:
| § | Create Arabidopsis lines using a vector that contains a Ceres’ constitutive promoter directing HAP1-VP16 expression as well as UASHAP1 — driven expression of a fluorescent protein (line A) | ||
| • | Create Arabidopsis lines using a vector that contains a “weak” cell/tissue specific promoter directing HAP1-VP16 expression as well as UASHAP1 — driven expression of a fluorescent protein (line B) | ||
| • | Create Arabidopsis lines using a vector that contains UASHAP1 — driven aequorin or a spectrally distinct fluorescent protein (line C) | ||
| • | Cross line C into lines A and B (or retransform construct from C into A or B using a novel, non-PPT based selectable marker) | ||
| • | Evaluate RNA levels, and demonstrate presence of transactivated protein function in a range of at least 10 cell types in statistically relevant sets of independent Arabidopsis transformants. |
| Transgene activation by HAP1 in monocots: the following milestones should be achieved by December 2003: |
| • | Create rice lines using a vector that contains a Ceres’ constitutive promoter directing HAP1-VP16 expression as well as UASHAP1 — driven expression of a fluorescent protein (line A) | ||
| • | Create rice lines using a vector that contains a “weak” cell/tissue specific promoter directing HAP1-VP16 expression as well as UASHAP1 — driven expression of a fluorescent protein (line B) | ||
| • | Create rice lines using a vector that contains UASHAP1 — driven aequorin or a spectrally distinct fluorescent protein (line C) | ||
| • | Cross line C into lines A and B (or retransform construct from C into A or B using a novel, non-PPT based selectable marker) | ||
| • | Evaluate RNA levels, and demonstrate presence of transactivated protein function in a range of at least 10 cell types in statistically relevant sets of independent rice transformants. |
Exhibit C
Exemplary Technological Elements
Exemplary Technological Elements
A polynucleotide sequence encoding a protein or polypeptide
A polynucleotide sequence regulating the expression of a coding sequence
A polynucleotide sequence regulating the transcription of a coding sequence
A polynucleotide regulating the stability of a transcript
A polynucleotide regulating the translation of a transcript
A polynucleotide sequence capable of suppressing the activity of another polynucleotide
A polynucleotide sequence capable of suppressing the activity of a polypeptide or protein
A process or method to transform a plant or a plant cell
A process or method to select a desirable transformant
SPONSORED RESEARCH AGREEMENT
This Sponsored Research Agreement (the “Agreement”), effective as of the 1st day of
June, 2000 (the “Effective Date”), is by and between The Chancellor, Masters and Scholars of The
University of Cambridge (“University”) and Ceres, Inc., a Delaware corporation (“Ceres”).
W I T N E S S E T H :
WHEREAS, University, as a center for academic knowledge and research and development, and
through the work of Xx. Xxxxxxxx and others, has expertise, information and technology rights in
the field of plant genomics and developmental biology;
WHEREAS, Xx. Xxxxxxxx is an employee of University and has special expertise and know-how in
screening transformed tissues for analyzing expression in certain cell types using Green
Fluorescent Protein (“GFP”);
WHEREAS, University is interested in performing certain research and development work for
Ceres, including the making and screening of certain transgenic plants of interest to one or both
Parties, and Ceres is willing to fund such research and development work, all subject to the terms
and conditions of this Agreement;
NOW, THEREFORE, in consideration of the foregoing premises and the following mutual covenants,
and other good and valuable consideration, the receipt of which is hereby acknowledged, and
intending to be legally bound hereby, the Parties agree as follows:
1. DEFINITIONS
For purposes of this Agreement the following terms shall have the following meanings:
1.1 “Affiliates” shall mean (a) any company owned or controlled to the extent of at least
fifty percent (50%) of its issued and voting capital by a Party to this Agreement and any other
company so owned or controlled (directly or indirectly) by any such company or the owner of any
such company, or (b) any partnership, joint venture or other entity directly or indirectly
controlled by, controlling, or under common control with, to the extent of at least fifty percent
(50%) of voting power (or otherwise having power to control its general activities), a Party to
this Agreement
1.2 “Background Technology” shall mean the Technologies developed prior to the Effective Date,
which the University or Ceres owns, or has license rights to, and which are useful for the Purpose.
The Party’s respective Background Technology shall be referred to as Ceres Background Technology
and University Background Technology. The University Background Technology shall include in
particular certain Patent Rights and Technology Rights relating to (i) Arabidopsis transgenic
plants and (ii) the hap1 and other extensin-gfp gene constructs. The Patent Rights related hereto
are listed in Exhibit D.
1.3 “Biological Materials” shall mean any plants, seeds, microorganisms, cells, parts of
cells, DNA, RNA, cDNA, proteins, peptides, enzymes, and any combination of the foregoing, and/or
other organic matter and/or other biologically active compounds.
1.4 “Biological Product(s)” means any product comprising the Biological Materials.
1.5 “University Patent Rights” means all Patent Rights owned or co-owned by University, but on
which no Ceres’ inventors are named.
1.6 “Ceres’ Patent Rights” means all Patent Rights owned or co-owned by Ceres.
1.7 “Confidential Information” means any information, disclosed by one Party to this Agreement
to the other Party, that has any commercial value to the disclosing Party’s business, research,
development or other activities. Confidential Information includes, without limitation,
inventions, biological materials, technical information, trade secrets, financial information,
product plans, customer lists, marketing plans and strategies, forecasts and other business
information, improvements, ideas, works of authorship, processes, computer programs, techniques,
schematics, data, gene sequences, gene expression data, protein sequences, protein structures,
regulatory sequences, and other data.
1.8 “Exclusive License Agreement” shall mean these or that certain exclusive license
agreement(s) to be negotiated between the Parties on fair and reasonable terms on commencement of
the Research Project.
1.9 “Other Project Technology” shall mean any Technology which the University develops which
is based on or created by using Project Technology and/or which furthers the Purpose without
funding or information from Ceres during the Research Project..
1.10 “Patent Rights” means all patents and patent applications throughout the world, including
any reissues, extensions, substitutions, continuations, divisions, and continuations-in-part
applications, reexaminations or extensions or other government actions which extend the life of a
patent, and all rights to apply for patent protection and all rights, if any, to xxx or bring other
actions for past, present or future infringement of such rights.
1.11 “Products” shall mean all products, processes, or services including Biological Products
that are to be commercialized, the manufacture, use or sale of which is covered by any claim of one
or more patents within the University Background Technology, and/or Project Technology.
1.12 “Party” means University or Ceres, collectively they are sometimes referred to as the
“Parties”.
1.13 “Project Technology” shall mean any and all Technology developed or obtained during and
resulting from the Research Project, but excluding any Background Technology and Other Project
Technology.
1.14 “Purpose” shall mean the generation and analysis and transfer to Ceres of data and large
numbers of transgenic Arabidopsis plants and/or seeds transformed with constructs
containing the GFP gene the expression of which is indirectly driven by a large amount of
different plant promoters, as further described in the Research Plan.
1.15 “Research Budget” shall mean the mutually agreed document attached hereto as Exhibit B
that specifies the funding for the Research Project.
1.16 “Research Funds” shall mean any funds paid or credited to the University by Ceres under
this Agreement as further specified in the Research Budget.
1.17 “Research Project” shall mean the collaborative research program under which University
and Ceres shall perform certain research and development activities in pursuit of the Purpose and
in accordance with the Research Plan. The Research Project shall continue until the earlier of (i)
the completion of the last task performed with the aid of the Research Funds, or (ii) the
expiration or termination of this Agreement.
1.18 “Research Plan” shall mean the mutually agreed document attached hereto as Exhibit A,
that describes the respective research experiments and the specific responsibilities of the Parties
in performing the Research Project.
1.19 “Technology” shall mean any Biological Material, Biological Products, data, methods,
protocols, procedures, processes and the like, and the Patent Rights and Technology Rights relating
thereto.
1.20 “Technology Rights” shall mean existing and future proprietary rights, including but not
limited to know-how rights, trade secret rights, copyrights, design rights, and all other
intellectual property rights (including without limitation the right, if any, to xxx or bring other
actions for past, present or future infringement of such proprietary rights), but excluding Patent
Rights.
2. THE RESEARCH PROJECT
2.1 Research Project. University and Ceres shall carry out the Research Project in
accordance with the Research Plan attached hereto as Exhibit A.
2.2 Research Plan. The Research Plan shall be signed by the Parties and shall include
a detailed outline of the Research Project, time schedules for performance of specific tasks, and
an annual budget. The Parties recognize that changes to the Research Plan may be required, and
therefore agree to negotiate in good faith the terms of amendments to the Research Plan.
Modifications to the Research Plan shall only become effective, however, through a written
amendment to the Research Plan executed by the Parties
2.3 The Parties agree to fully cooperate, including making their respective personnel
available at reasonable times, in order to expedite carrying out the Research Project efficiently
and avoiding unwarranted expenditure of effort.
2.4 Research Performed by University.
2.4.1 University shall use all reasonable efforts to carry out its responsibilities under the
Research Project in accordance with the Research Plan, and within the timetables set forth therein.
2.4.2 University shall allocate its resources substantially as set forth in the Research Plan
including, but not limited to, providing at least three (3) full time equivalent personnel
(“FTEs”), who shall work on the Research Project. At least two (2) of the FTEs shall be at least
at the postdoctoral level. The other one (1) FTE shall be the technician level.
2.4.3 The principal scientist or investigator who will direct the performance of the Research
Project on behalf of University is, unless the Parties otherwise agree, Xx. Xxxxx Xxxxxxxx. All
inventions and research information disclosed pursuant to this Agreement, and all other
communications concerning the Research Project shall be directed to Xx. Xxxxx Xxxxxxxx.
2.4.4 Xx. Xxxxxxxx and his team shall not, during the conduct of the Research Project, engage
in any research in pursuit of the Purpose and the Research Project to which any other commercial
entity would receive rights.
2.5 Research Performed by Ceres.
2.5.1 In order to accelerate and facilitate the Research Project, Ceres may, in its sole
discretion:
2.5.1.1 provide certain of its Background Technology to University and provide certain
Confidential Information relating thereto to University, and
2.5.1.2 perform analysis on Project Technology provided by University.
2.5.2 Any results arising from work performed under this Section 2.5 shall be deemed to be
Project Technology.
3. CONSIDERATION
3.1 Research Funds. Ceres shall provide Research Funds over a three-year period to
support the Research Project, in accordance with the Research Budget attached hereto as Exhibit B.
The Research Funds paid under this Agreement shall not exceed £ (English pounds) 575,478 in total.
Installments of the Research Funds shall be provided on a quarterly basis with the first payment
occurring within thirty (30) days from the signature date of this Agreement and thereafter within
thirty (30) days from the start of each quarter. The foregoing notwithstanding, the Parties may,
from time to time, discuss the scope, direction, and pace of the Research Project, and Ceres may
agree to provide greater amounts of funding if University agrees to commit greater resources to the
Research Project.
3.2 Equipment. On the expiration or termination of this Agreement, the University
shall retain title to any equipment provided by Ceres, or purchased with funds provided by Ceres,
under this Agreement.
4. OWNERSHIP
4.1 Background Technology. For the avoidance of doubt, each Party shall have and
retain all rights, title and interest to its respective Background Technology used in the Research
Project.
4.2 Project Technology. Ceres shall be the sole owner of all right, title and
interest to Project Technology. University hereby assigns to Ceres all of its rights, title and
interest to the Project Technology. . If University is prevented by law or otherwise prevented
from assigning any Project Technology to Ceres, University hereby grants to Ceres all licenses, to
the fullest extent possible, to effectuate the intent of this Agreement that Ceres be assigned all
Project Technology, and to otherwise effectuate the purposes of this Agreement.
4.3 Other Project Technology. University shall have and retain all rights, title and
interest to the Other Project Technology.
5. PATENT RIGHTS
5.1 University Patent Rights. Subject to all of the terms and conditions of the
Exclusive License Agreement, University will diligently prosecute and maintain the patents and
patent applications throughout the world comprising University Patent Rights covering University
Background Technology and Other Project Technology or relating to any other technology exclusively
licensed to Ceres under the Exclusive License Agreements. To aid in effectuating the purposes of
this Agreement and the Exclusive License Agreement, University shall use all reasonable endeavors
to ensure that each University’ inventor of Patent Rights covering Project Technology shall do all
acts and execute documents as may be necessary by patent laws to give effect of this clause 5.1.
5.2 Ceres Patent Rights. Subject to all of the terms and conditions of the Exclusive
License Agreement, Ceres will diligently prosecute and maintain the patents and patent applications
throughout the world comprising Ceres Patent Rights arising out of the Research Project. In the
event patent laws require the naming of University inventors as co-inventors on Ceres’ Patent
Rights, University will assign its ownership rights of these Patent Rights to Ceres. University
shall use all reasonable endeavors to ensure that each University’ inventor of Patent Rights
covering Project Technology shall do all acts and execute documents as may be necessary by patent
laws to give effect of this clause 5.2
6. EXCLUSIVE LICENSE AGREEMENT
During the period of negotiation of the Exclusive License Agreement neither Xx. Xxxxxxxx nor
any member of his team or any person appointed to represent the University on behalf of Xx.
Xxxxxxxx or his team shall enter into any arrangements with third parties which may conflict with
the Results, the field of research or terms of this Agreement.
7. DISCLOSURE OBLIGATIONS
7.1 Disclosure to Ceres. Upon execution of this Agreement University shall promptly
disclose in writing or other tangible form to Ceres all of University Background Technology
existing as of the Effective Date which University has already used or plans to use in the
Research Project.
7.2 Disclosure during Research Project. During the term of the Agreement University
shall promptly disclose to Ceres any and all Project Technology as it arises, in carrying out the
Research Project. Immediately after University becomes aware of the existence of any Other Project
Technology, University shall disclose such Other Project Technology to Ceres.
7.3 Means of Disclosure. Any disclosure as required by this Section 7 shall be made
in tangible forms, which are mutually acceptable to both Parties. To facilitate such transfer, the
Parties shall agree upon mutually acceptable means of accomplishing such end, which means shall
include correspondence via telephone, mail, e-mail and fax as well as meetings at least every six
(6) months during the term. Such meetings shall be held at the University facilities or at Ceres,
or at such other locations as may be mutually agreed upon. About two (2) weeks prior to each such
meeting, the University shall provide Ceres with written reports concerning the Research Project.
All costs incurred in undertaking all communications relating to the Research Project shall be
borne by Ceres. Ceres shall reimburse the University for reasonable travel costs incurred in
traveling to Ceres or other locations selected by Ceres. All transportation will be coach class or
equivalent. The University or its employees shall itemize each expense and provide Ceres with
receipts for all expenses that are to be reimbursed.
7.4 Mutual Non-Disclosure Obligations. Each Party hereby agrees that it shall keep
confidential and not use for any purpose, except as provided herein, all Confidential Information
supplied to it (the “Recipient”) by the other Party (the “Disclosing Party”) during the term of
this Agreement and for five (5) years after termination or expiration hereof; provided, however,
that the foregoing obligations of confidentiality and non-use shall not apply to the extent that
any Confidential Information is demonstrated by written records to be (a) already known to the
Recipient or one of its Affiliates at the time of disclosure hereunder (provided the Recipient
and/or its Affiliates comply with any restrictions imposed by third parties) or is hereafter
developed by the Recipient or one of its Affiliates in the course of work entirely independent of
any disclosure hereunder; or (b) publicly known prior to or after disclosure hereunder other than
through acts or omissions of the Recipient or one of its Affiliates; or (c) disclosed in good faith
to the Recipient or one of its Affiliates by a third party (provided the Recipient and/or its
Affiliates comply with any restrictions imposed by third parties). This does not prevent
disclosure to third parties by the Recipient under a secrecy or confidentiality agreement with
essentially the same confidentiality provisions provided herein in connection with the exercise of
its rights under this Agreement (but only to the extent permitted herein). In addition, disclosure
may be made (i) to Recipient’s employees, consultants, representatives, agents and advisors
provided that such persons are subject to confidentiality obligations consistent with the ones set
forth in this Section 6.5, and (ii) to governmental agencies to the extent required to secure
governmental approval for the Products; provided, however, that the Recipient shall seek to limit
disclosure and to obtain confidential treatment therefor.
7.5 Affiliates, Licensee and Sublicensees. Nothing herein shall be construed as
preventing Ceres from disclosing any information received from the University to an Affiliate,
licensee or to a sublicensee of Ceres, provided such Affiliate, licensee or sublicensee has
undertaken a similar obligation of confidentiality with respect to the Confidential Information.
7.6 Publication and Patentability. In order to avoid the loss of, or the diminution
in value of, valuable patent rights the University agrees to ensure that the University, the
University’ inventors of the Patent Rights covering Project Technology and other University
employees and consultants involved in the Research Project delay the dissemination or publication
of any information developed in the course of performance of the Research Project or based upon any
Ceres information or Other Project Technology until thirty (30 ) days after Ceres has been
furnished with the full text of any proposed dissemination or publication. If Ceres determines
that such dissemination or publication would jeopardize any Patent Rights, Ceres shall notify the
University in writing within the thirty (30 ) day period and may elect in such notice to delay the
proposed publication for a further period, not to exceed ninety (90) days in order to protect
potential Patent Rights. Xx. Xxxxxxxx agrees to comply with the foregoing and to use his
reasonable efforts to ensure that all University employees working on the Research Project comply
with the foregoing.
7.7 Deposit of Seeds. In order to avoid the loss of, or diminution in value of,
valuable patent rights and other proprietary rights, should the University choose to transfer seeds
to national stock centers, such transfers shall be conditioned upon (i) such national stock center
not releasing the deposited seeds for a period of 18 months from the date on which Ceres was
provided with such seeds (such period, for each seed type, the “Lead Period”), (ii) the national
stock center agreeing not to release such seeds to any third party unless such third party executes
in advance, the attached Material Transfer Agreement TYPE A, including the restriction that the
third party shall not use the seeds for commercial purposes and (iii) that copies of all executed
Material Transfer Agreements related to such seeds are promptly provided by University to Ceres.
7.8 Disclosure to Collaborators. Xx. Xxxxxxxx may choose to transfer selected seed
items and associated information to a few special collaborators, listed in Exhibit E, which can be
updated by Xxx Xxxxxxxx and which updates will be communicated to Ceres in writing, during the Lead
Period applicable to such seed type, provided that the collaborating Party executes in advance the
attached Materials Transfer Agreement TYPE B and provided that (i) all relationships of such
collaborators with commercial entities be disclosed to Ceres, in writing, prior to the transfer of
any materials, and (ii) Ceres is provided with a copy of the executed Material Transfer Agreements
promptly after they are signed.
7.9 Press Releases. All press releases which one Party desires to make relating to
the Research Project or any of the matters contemplated hereunder must be prepared by such Party as
a joint press release of the Parties and must not be publicly released or released to the press
without the prior written consent of the other Party.
7.10 Use of Tradenames. Neither Party shall disclose or use the name of the other for
any purpose without the prior written consent of the named Party, except for the purposes of
referring to this Agreement in disclosures to be made in documents in connection with financings
and/or as required by law.
7.11 Internet Communications. To the extent that the Parties use the internet as a
means of communication, all e-mail and/or other internet based communications containing
Confidential Information shall be encrypted.
8. REPRESENTATIONS AND WARRANTIES
8.1 Ceres Representations and Warranties. Ceres represents to the University and the
University’ inventors of Patent Rights that:
8.1.1 Ceres is a corporation duly organized, validly existing and in good standing under the
laws of the State of Delaware and has all requisite corporate power and authority to carry on its
business as now conducted; and
8.1.2 All corporate action on the part of Ceres and its officers and directors necessary for
the authorization, execution and delivery of this Agreement and the performance of all obligations
of Ceres hereunder has been taken, and this Agreement constitutes a valid and legally binding
obligation of Ceres, enforceable in accordance with its terms.
8.2 University Representations and Warranties.
8.2.1 University is an institution of higher learning and research duly organized, validly
existing and in good standing under the laws of England and Wales has all requisite power and
authority to carry on its business as now conducted;
8.2.2 All action on the part of University necessary for the authorization, execution and
delivery of this Agreement and the performance of all obligations of University hereunder has been
taken, and this Agreement constitutes a valid and legally binding obligation of University,
enforceable in accordance with its terms; and
8.3 Disclaimers.
8.3.1 Whilst the Parties will use all reasonable endeavors to ensure the accuracy of the work
performed and any information given in performance of this Agreement, the Parties make no warranty,
express or implied, as to the accuracy of its work under the Research Project or the results or any
other advice or information furnished by it or by any of its employees, students, agents or
appointees who work on the Research Project
8.3.2 The Parties accept no responsibility whatsoever for any use which may be made of any
work carried out under or pursuant to this Agreement, of the Project Technology, or of its
Background Technology, and no liability whatsoever either direct or indirect shall rest upon a
Party, its employees, students, agents or appointees for the effects of any product or process that
may be developed, manufactured, used, sold, imported or distributed by or on behalf of the other
Party or any Affiliate or sublicensee of the other Party, notwithstanding that such product or
process may be based upon the findings of the Research Project, the results or upon any other
advice or information furnished by a Party, its employees, students, agents or appointees under
this Agreement.
8.3.3 Other than the above warranties, none of the Parties to this Agreement make any
warranties or representations, whether express or implied.
9. LIMITATION OF LIABILITY
9.1 EXCLUSIONS. IN NO EVENT WILL ANY PARTY BE LIABLE UNDER ANY CONTRACT, NEGLIGENCE,
STRICT LIABILITY OR OTHER THEORY FOR ANY INDIRECT, INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES
INCLUDING, BUT NOT LIMITED TO, LOSS OF REVENUES AND LOSS OF PROFITS IN CONNECTION WITH THIS
AGREEMENT OR IN CONNECTION WITH THE USE OF THE INVENTION(S), BIOLOGICAL MATERIALS, PATENT RIGHTS,
PROPERTY RIGHTS OR PRODUCTS.
9.2 Limitation. Under no circumstances whatsoever shall the University’s liability to
Ceres under or otherwise in connection with this Agreement exceed sums paid by Ceres to the
University under this Agreement together with the Exclusive License Agreement.
10. INDEMNIFICATION
10.1 Ceres Indemnity. Ceres agrees to indemnify, hold harmless defend and hold
harmless the University, its employees, students, agents and appointees, including but not limited
to, Xx. Xxxxxxxx, from and against any and all liability, loss, damage, cost or expense (including
reasonable legal fees, court costs and other expenses of litigation) arising out of or in
connection with third party claims relating to:
10.1.1 use of Ceres’ Background Technology or Project Technology in accordance with the terms
of this Agreement;
10.1.2 any alleged infringement of a third party’s intellectual property rights by reason of
Ceres’ activities in relation to the Research Project or this Agreement; or
10.1.3 any Product or process developed, manufactured, used, sold, imported or distributed by
or on behalf of Ceres, its Affiliates or sublicensees arising out of the Research Project or in any
way out of this Agreement.
11. TERM AND TERMINATION
11.1 Term. This Agreement shall come into effect on the Effective Date and shall
continue, unless earlier terminated or extended in accordance with this Agreement, for three (3)
years from the Effective Date. For purposes of this Section 11, University shall be deemed to be
one Party and Ceres the other Party. Xx. Xxxxxxxx shall have no individual rights of termination.
11.2 Termination upon Breach. Upon breach or default of any of the terms and
conditions of this Agreement, the defaulting Party shall be given notice of such default in writing
and a period of thirty (30) days after receipt of such notice to correct the breach or default. If
(a) the default or breach (i) is material to this Agreement taken as a whole, and (ii) is not
corrected within said thirty (30) day period and the defaulting Party has not taken reasonable
steps to cure the same, and (b) the Party not in default has fully complied with all of its
obligations under this Agreement and (c) the Party not in default has no adequate remedy from
monetary changes, the Party not in default shall have the right to terminate this Agreement.
11.3 Termination upon Bankruptcy. A Party shall have the right to terminate this
Agreement upon the first to occur of the following events: (i) a petition of action is filed or
action taken by or against the other Party under any law dealing with insolvency or bankruptcy;
(ii) a receiver is appointed over the assets or undertaking of the other Party; (iii) the other
Party enters into a deed of arrangement or makes an assignment for the benefit of creditors; or
(iv) the other Party ceases to function as a going concern or an order is made or a resolution
passed to that effect.
11.4 Ceres Termination Rights. In addition to the above termination rights, Ceres
shall be entitled to terminate this Agreement as follows:
11.4.1 If Xx. Xxxxxxxx leaves University during the term of this Agreement, and is not
replaced within sixty (60) days thereafter with a principal scientist or investigator acceptable to
Ceres in its sole discretion, Ceres shall have the right to terminate this Agreement by written
notice to University.
11.4.2 Ceres believes that the intended objectives of the Research Project are not being
achieved to a reasonable extent because of a major lack of due performance by University (hereafter
referred to as “Lack of Diligence”), Ceres may, in writing, demand adequate assurance of such due
performance. If University does not provide such assurance in all material respects within sixty
(60) days after receiving such demand, Ceres shall have the right to terminate this Agreement at
any time by giving written notice thereof to the other Party.
12. MISCELLANEOUS
12.1 Reserved Rights. Ceres may during or after the Term of this Agreement
independently perform any research, including, without limitation, research related to the purpose.
University retains the right, after the Research Project is complete, to perform research and
development work in the field of the purpose independent of Ceres.
12.2 Mediation and Governing Law.
12.2.1 If any dispute arises out of or in connection with this Agreement the Parties will
attempt in good faith to settle it by negotiation.
12.2.2 If the Parties are unable to settle any dispute by negotiation within twenty-eight (28)
days the Parties will attempt to settle it by mediation in accordance with the Centre for Dispute
Resolution (CEDR) Model Mediation Procedure.
12.2.3 To initiate a mediation a Party must give notice in writing to the other Party
requesting a mediation in accordance with Section 12.7.
12.2.4 This Agreement and all questions of construction, validity and performance under this
Agreement shall be governed by English Law.
12.3 Independent Contractors. Nothing in this Agreement is intended or shall be
deemed to constitute a partnership, agency, employer-employee or joint venture relationship between
the Parties. All activities by the Parties hereunder shall be provided as independent
contractors. Neither Party shall incur any debts or make any commitments for the other except
to the extent, if at all, specifically provided herein.
12.4 Force Majeure. If the performance of any part of this Agreement by a Party is
prevented, restricted, interfered with or delayed by reason of any cause beyond the reasonable
control of the Party liable to perform, the Party so affected shall use its diligent efforts to
avoid or remove such causes of non-performance and shall continue performance with the utmost
dispatch whenever such causes are removed. When such circumstances arise, the Parties shall
discuss what, if any, modification of the terms of this Agreement may be required in order to
arrive at an equitable solution.
12.5 Construction. In the event any portion of this Agreement shall be held illegal,
void or ineffective, the remaining portions hereof shall remain in full force and effect, as long
as it does not materially alter the purpose and performance of this Agreement. If any of the terms
or provisions of this Agreement are in conflict with any applicable statute or rule of law, then
such terms or provisions shall be deemed inoperative to the extent that they may conflict therewith
and shall be deemed to be modified to conform with such statute or rule of law. In the event that
the terms and conditions of this Agreement are materially altered as a result of this Section, the
Parties will renegotiate the terms and conditions of this Agreement to resolve any inequities.
12.6 Entire Agreement. This Agreement together with the Exclusive License Agreement
constitutes the entire agreement between the Parties relating to the subject matter hereof and
supersede all prior agreements, understandings, writings, and discussions between the Parties
relating to said subject matter. No terms or provisions of this Agreement shall be varied or
modified by any prior or subsequent statement, conduct or act of either of the Parties, except that
the Parties may amend this Agreement by written instruments specifically referring to and executed
in the same manner as this Agreement.
12.7 Notices. All notices pertaining to this Agreement shall be in writing and sent
by two-day delivery via an internationally recognized delivery service to the other Party at the
following addresses or such other address as such Party shall have furnished in writing to the
other Party in accordance with this Section 12.7:
For the University:
Xxx Xxx Xxxxxxx Xxxxxxx XxxxXxxxxxxxx XX0XX
Great Britain
Attention: Head of Research Grants and Contracts
For Ceres:
A notice shall be deemed to have been received on the day after deposit with the delivery
service, if sent by overnight delivery.
12.8 No Third Party Beneficiaries and No Assignment. This Agreement shall be binding
upon and inure to the benefit of the successors in interest of the respective Parties. This
Agreement shall not be assigned by any Party without the written consent of the other Party;
provided, however, Ceres may assign this Agreement to any Affiliates or to any corporation with
which it may merge or consolidate, or to which it may transfer all or substantially all of its
assets or business, without obtaining the consent of the other Party.
12.9 Further Assurances. Each Party hereto shall execute such further papers or
agreements as may be necessary to effect the purposes of this Agreement and carry out its
provisions.
12.10 Export Control Laws. The Parties will observe all applicable National laws and
foreign laws with respect to the transfer of materials and related technical data to foreign
countries, including, without limitation, the International Traffic in Arms Regulations (ITAR) and
the applicable export control regulations.
12.11 No Waiver. The failure of either Party at any time or times to require
performance of any provision hereof shall in no manner affect its right at a later time to enforce
the same. No waiver by either Party of any condition or term in any one or more instances shall be
construed as a further or continuing waiver of such condition or term or of any other condition or
term.
12.12 Headings. The headings of the several sections are inserted for convenience of
reference only and are not intended to be a part of or to affect the meaning or interpretation of
this Agreement.
12.13 Survival. Upon termination of this Agreement for any reason, Sections 4, 5, 6,
7, 8.4, , 9,, 11 and 12 and any accrued rights, obligations and causes of action shall survive
termination of this Agreement.
IN WITNESS WHEREOF the respective Parties hereto have executed this Sponsored Research
Agreement by their duly authorized officers as of the Effective Date.
| THE UNIVERSITY OF CAMBRIDGE | CERES, INC. | |||||||
By |
||||||||
| By: | /s/ Xxxx Xxxxx | |||||||
| Title Director of Research Services | ||||||||
| Title Chief Operating Officer | ||||||||
| XX. XXXXX XXXXXXXX |
||||||||
| Signature: /s/ Xxxxx Xxxxxxxx |
||||||||
Title University Lecturer |
||||||||
Exhibit A
Research Plan
Decoding gene expression and plant cell fate.
Xx. Xxx Xxxxxxxx,
Department of Plant Sciences, University of Cambridge,
Xxxxxxx Xxxxxx, Xxxxxxxxx. XX0 0XX.
Tel: x00-0000-000000, Fax: x00-0000-000000,
Email: xx000@xxx.xx.xx, web site: xxxx://xxx.xxxx0X.xxx
Department of Plant Sciences, University of Cambridge,
Xxxxxxx Xxxxxx, Xxxxxxxxx. XX0 0XX.
Tel: x00-0000-000000, Fax: x00-0000-000000,
Email: xx000@xxx.xx.xx, web site: xxxx://xxx.xxxx0X.xxx
Objectives of the proposed research.
The genetic control of plant development is mediated by cellular interactions, and an exchange
of positional information contributes to the self-organisation and coordination of cells during
development. The Arabidopsis root meristem provides an ideal test-bed for probing these
interactions. The root meristem grows indeterminately, is genetically amenable, has a simple and
transparent architecture, and can be induced to form de novo in adult tissues. We have developed
new genetic and optical techniques for visualising and manipulating cells within living meristems,
using a modified green fluorescent protein (GFP).
With these new approaches, it is crucial to obtain GAL4-independent markers for precisely
monitoring cell fate, and to understand the patterns of gene expression that underlie different
cell fates. I believe that recent technical advances will allow both of these objectives to
be simply realised. The first part of this proposal describes our general approach and new
methods that we have developed. The second part describes a high throughput scheme for rapidly
generating a library of Arabidopsis lines that express a cell wall localised form of GFP in
specific cell types. These enhancer trap lines will contain a novel transcription factor based on
HAPI, similar to a GAL4-based strategy that we have already developed.
The enhancer trap lines will:
(i) provide GAL4-independent GFP markers for cell fate, (Ii) allow precise
HAP1-dependent transactivation of genes in Arabidopsis,
(iii) allow histological clearing of GFP expressing tissues for detailed 3D mapping of
cell arrangements in meristems and during embryogenesis, and
(iv) the presence of a new fluorescent cell wall epitope will allow the simple isolation of highly
specific cell types in relatively large quantities for gene expression analysis using microarrays
or DNA chips and protein and biochemical analyses.
| Page 1 |
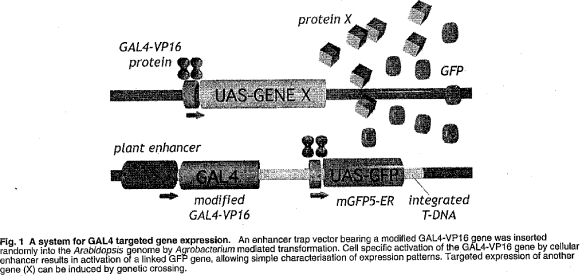
Background.
Developing multicellular tissues or organs generally demonstrate a capacity for
self-organisation. For example, wounded tissues generally respond in a robust and coordinated
fashion to allow repair, and local induction events can initiate prolonged and coordinated
developmental processes. These types of developmental plasticity and functional autonomy are
particularly evident in plant tissues. The basic features of a plant’s body plan are established
during embryogenesis, however its final form results from the continued growth of meristems and the
formation of organs throughout its life, often in a modular and indeterminate fashion. Plant cells
are constrained by rigid cell walls and are generally non-motile, so there is the clear possibility
that cell fates within a meristem are determined by lineage. However, evidence from plant chimera
and wounding studies have demonstrated a more important role for cell-cell interactions during fate
determination (reviewed in Xxxxxxx & Sussex, Patterns in Plant Development, 1989) and
laser ablation of cells within the Arabidopsis root meristem has shown that after the death of a
cell, a neighbouring cell can be triggered to divide and compensate for the loss (van der Xxxx et
al., Nature 37Q:62-65, 1995). It is likely that positional information
during plant development is obtained via cell-cell contact, and that the coordination and fate of
cells within a developing meristem may be determined by a network of local cellular interactions.
We have chosen the Arabidopsis root meristem as a model system for investigating intercellular
interactions. The root meristem possesses indeterminate growth and has a simple and transparent
architecture. Arabidopsis is genetically amenable, and one can routinely generate transgenic lines
for work with the intact organism.
In order to dissect local cell-cell interactions it is crucial that we can (i) clearly image
individual cells inside living meristems and (ii) have the means to perturb them.
| Page 2 |
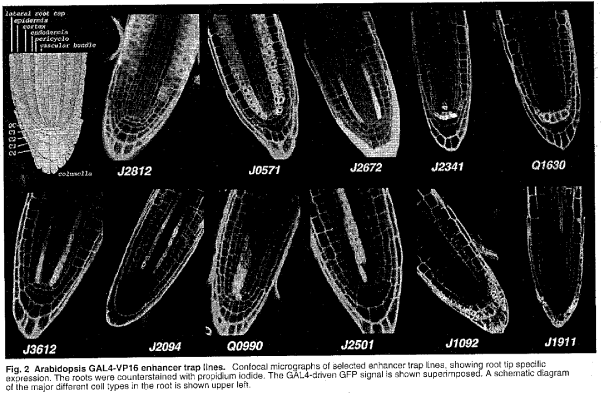
Over the past several years, we have developed a set of new genetic and optical techniques which
allow us to visualise and manipulate cells within living plants.
(i) Visible gene reporters.
The jellyfish green fluorescent protein (GFP) has been adapted for use as a bright marker in
transgenic plants. The wild-type GFP cDNA is not expressed in Arabidopsis. We have extensively
modified the gfp gene to remove a cryptic intron, to introduce mutations that confer improved
folding and spectral properties and to alter the subcellular localisation of the protein. All of
these alterations have been incorporated into a single modified form of the gene (mgfp5-ER) which
we now routinely use for monitoring gene expression and marking cells in live transgenic plants
(Xxxxxxxxx et al., Current Biology §: 1653-1663, 1996; Xxxxxxxx et al., PNAS 94:2122-2127, 1997).
(ii) Multispectral dynamic imaging.
We have developed fluorescence microscopy techniques for high resolution observation of living
cells. The expression of GFP within an organism produces an intrinsic fluorescence that colours
normal cellular processes, and high resolution optical techniques can be used non-invasively to
monitor the dynamic activities of these living cells. Using coverslip-based culture vessels,
specialised microscope objectives and the optical sectioning properties of the confocal microscope,
it is possible to monitor simply and precisely both the arrangement of living cells within a
meristem, and their behaviour through long time-lapse observations (see
xxxx://xxxxxxx0.xxxxxxxx.xxx.xx.xx). We have recently constructed cyan and yellow emitting GFP
variants that can be distinguished from the green fluorescent protein during confocal microscopy.
These colour variants have enabled simultaneous imaging of different tagged proteins in living
cells.
| Page 3 |
(iii) GAL4 targeted GFP expression.
In order to genetically manipulate cells during meristem development, we have devised a scheme
for targeted gene expression, which is based on a method widely used in Drosophila (Brand and
Perrimon, Development 118:401-415, 1993). We have used an “enhancer-trap” strategy to generate many
transgenic plants which express different patterns of a yeast transcription activator, GAL4. A
chosen target gene can then be placed under the control of GAL4 upstream activation sequences
(VAS), transformed into plants, and maintained silently in the absence of GAL4. Genetic crosses
between this single line and any of the library of GAL4-containing lines specifically activates the
target gene in a · particular tissue or cell type (Fig. 1). The phenotypic consequences of
mis-expression, including those deleterious to the organism, can be conveniently studied.
We found that GAL4 is not expressed in Arabidopsis due to a high A/T content, which can interfere
with mRNA processing in plants. We have produced a modified form, mGAL4-VPI6, that it is expressed
efficiently in plants, and randomly inserted the modified gene into the Arabidopsis genome, using
Agrobacterium tumefaciens-mediated transformation. The transformation vector was designed so that
expression of the mGAL4-VP16 gene would be dependent upon the fortuitous proximity of an
Arabidopsis enhancer element. The inserted DNA also contained a GAL4-responsive mGFP5-ER gene.
Thus, interesting “enhancer-trap” patterns of GAL4 gene expression were immediately and directly
visible, with each GAL4-expressing cell marked by bright green fluorescence. We have used in vivo
detection of GFP to directly screen for GAL4-directed GFP expression in 8000 regenerated plantlets.
We have documented a collection of over 250 Arabidopsis lines with distinct and stable patterns of
mGAL4-VPI6 and GFP expression in the root. These lines provide a valuable set of markers, where
particular cell types are tagged and can be visualised with unprecedented ease and clarity in
living plants (Fig. 2).
(iv) Targeted misexpression.
Most importantly, mGAL4-VP16 expression within these lines can be used to direct the
expression of a chosen gene at a precise time and place within the organism. We have produced
transgenic plants which maintain regulatory proteins or toxins, silent behind a GAL4-responsive
promoter. We can now activate these genes in specific cells by crossing to a chosen mGAL4-VPI6
expressing line. For the first time, we have a system with the potential to both precisely perturb
and to monitor the behaviour of particular cells within a living plant. For example, we are (i)
using GAL4dependent expression of the A-chain of diphtheria toxin (DTA) to kill specific cells
within the root meristem, (ii) driving misexpression of cell cycle regulatory proteins, to activate
or inhibit particular cell divisions within the root meristem (in collaboration with Xx. Xxx
Xxxxxxxx, University of York), and (iii) triggering ectopic expression of homeodomain proteins in
order to affect cell fate determination. In order to better interpret these experiments, it is
crucial to gain an improved understanding of the precise timing and arrangement of gene expression
and cell architecture within normal meristems, and in these genetically perturbed tissues.
Accordingly, we have developed better techniques for the three dimensional visualisation of cell
arrangements within meristems.
(v) 3D visualisation.
The architecture of primary meristems is established early, during embryogenesis.
Unfortunately, this process is occluded by the silique and ovary walls, making direct live
observations difficult. The 3D arrangements of plant cells can be observed using either physical or
optical sectioning techniques.
| Page 4 |
However the laborious nature of thin sectioning, the problem of obtaining the desired plane of
section, and difficulty of obtaining a complete series of sections has limited its use somewhat to
the skilled and patient. Optical sectioning has many advantages from the point of view of speed and
simplicity, and it can allow the direct viewing of living wholemounts. Here, transverse
sections need to be reconstructed from a series of Z-axis images. Nomarski optics have proved
useful for examining details within living tissues, but do not provide sufficient contrast and
resolution to allow precise 3D reconstruction of cell arrangements. Confocal laser scanning
microscopy provides a substantial improvement, but it has still proved difficult to optically
section deep into tissues due to light scattering and spherical aberration caused by particulate
subcellular matter and layers of refractile cell walls. I have been struggling with this problem
for some time, and have recently found some solutions.
Periodic acid treatment of carbohydrates produces aldehyde groups which can be reacted with various
fluorescent pseudoSchiff reagents. If fixed plant tissue is treated in this way, cell walls (and
starch-containing plastids, if present) become intensely and covalently labeled with the chosen
fluor. The tissue can then be directly cleared in a high refractive index agent containing chloral
hydrate, and mounted for microscopy. The combination of high levels of fluorescence and high
refractive index mountant allows the collection of extended Z-series images at very fine resolution
(0.2 -0.5 µM steps), using minimum confocal aperture, and without fear of photobleaching or signal
and resolution loss due to spherical aberration. The depth of image collection is limited mainly by
the working distance of the objective (>200JlM), and this allows simple optical sectioning
throughout an entire Arabidiopsis root at high resolution. In fact every cell within a
mature Arabidopsis embryo can be clearly visualised (Fig. 3). We now face the exciting
prospect of being able to clearly visualise the relative arrangement of every cell during meristem
initiation and development, and to be able to accurately map the order and pattern of cell
proliferation during meristem development.
We can routinely reconstruct the cellular structure of entire meristems for various experiments.
The large data files, between 100MB and 200MB in size, allow excavation of the data, production of
sections in arbitrary planes, and rendering of surface features. We can also use computer
visualisation methods, borrowed from the medical imaging field, to reduce large data sets to a
simple description of the 3D shapes and arrangement of cells in a meristem. These advanced software
methods for 3D segmentation allow visualisation of the dimensions, shapes and relative arrangements
of cells within optically sectioned
| Page 5 |
meristems (Fig. 4). These same methods can be used to analyse meristems that have been genetically
perturbed by GAL4 targeted cell ablation or misexpression.

(vi) Naturally insolubilised GFP marker.
With the techniques that we have already developed, one can readily visualise living
GFP-expressing cel1s, do precise 3D analysis of fixed and stained tissues and use the GAL4 system
for targeted misexpression experiments. These experiments have led us to look for better
GAL4-independent cel1 markers, and to search for ways of retaining the GFP signal in cleared
tissues for detailed 3D analysis of marked cel1s. (The 3D visualisation methods described above
result in bleaching and loss of GFP from cleared, stained tissues). Accordingly, we have recently
developed a natural1y insolublised form of GFP.
We have fused a variant of GFP to the coding sequence of a carrot extensin. Expression of this gene
fusion in transgenic Arabidopsis tissues results in the decoration of cel1 wal1s with bright
fluorescence (Fig. 5). Extensin becomes covalently linked to the cel1 wall matrix, and the
GFP-extensin marker is resistant to various clearing techniques that normal1y result in complete
loss of the protein from treated tissues. For example, the cel1 wall bound signal is retained after
complete alcohol dehydration or glycerol infiltration. In addition, the externalised GFP provides
an epitope tag that will be useful for the physical sorting of cells using simple immunological
methods. These properties would provide a substantial benefit for the screen outlined below.
PROPOSAL
We have constructed a novel enhancer trap vector that will allow the generation of stable
Arabidopsis lines with a robust fluorescent marker within specific cell walls. It will be possible
to see developing cells, deep in cleared embryonic and meristem tissues. The marker will also
provide a cell surface epitope. For the first time, it will be possible both to
visualise the precise 3D arrangements of different cell types and to simply and rapidly isolate
those same cell types for genetic or biochemical analysis.
1. HAP1-VP16 enhancer trap vector.
The yeast GAL4 protein is a member of a family of zinc-finger (Cys4) transcription factors
which are limited to fungi, and homologues have not been found in plants to date. In order to
generate a GAL4-independent system for targeted gene expression and generation of cel1 markers, we
have constructed a synthetic HAPI-VP16 gene. HAPI is another yeast zinc-finger transcription factor
related to GAL4, but with a different binding specificity. Yeast genes have a high A/T content and
are often poorly expressed in Arabidopsis due to aberrant post-transcriptional processing.
Therefore we have constructed a synthetic gene which has an elevated G/C content, and in which the
DNA binding domain is fused to the highly active and G/C-rich transcription activator domain of
VP16. We have also synthesised an optimised
| Page 6 |
multimeric binding site for HAP1, and cloned this behind a GFP reporter (Xxxxxx Xxxxx &
J.R., unpublished results). These elements have been used for the construction of an enhancer
trap vector, following the methods proven for the GAL4-based vector. We are in the process
of biologically testing this construction at present.
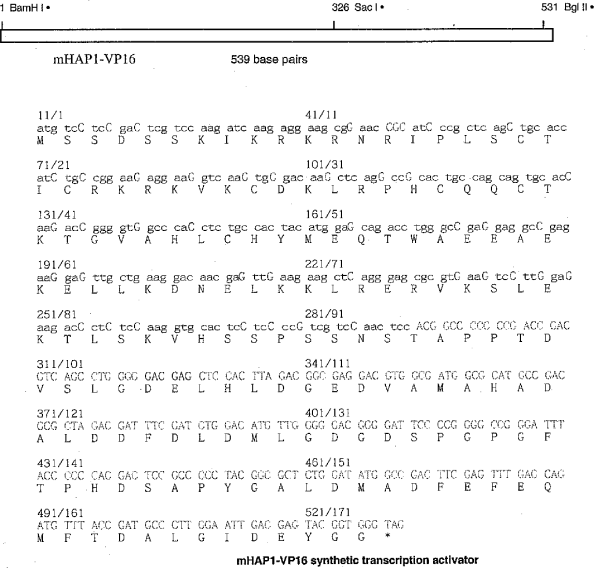
If this construction proves active, we will use this as the basis for a HAPI driven,
extensin-GFP enhancer trap screen. If verification of the HAPI vector is delayed for any reason, we
will use the proven GAL4-based vector. The extensin-GFP reporter gene will be inserted into the
appropriate enhancer trap vector and used to generate transgenic Arabidopsis lines.
2. High throughput screen.
All necessary reagents and techniques for a new enhancer trap screen are in place. We have
proven the feasibility of using: a cell wall GFP marker, a transcription factor based enhancer trap
screen, epifluorescence techniques for rapid screening and image documentation of primary
transformants,
| Page 7 |
confocal microscopy techniques for precise 3D visualisation of GFP expression patterns, and have
generated hundreds of highly specific GFP tagged Arabidopsis lines. At this point, the main
limitation to using cell wall tagged lines for cell sorting or misexpression experiments, is the
new screen itself. From a practical point of view, this is the most important part of this proposal
and is based upon our experiences with the earlier GAL4-GFP screen.
For speed, I propose that we use an Arabidopsis root transformation based protocol for the
generation of transgenic plantlets. In each experiment, large numbers of transgenic calli can be
regenerated over the course of a few weeks, induced to form shoots and roots, and directly screened
by epifluorescence microscopy for extensin-GFP expression in the developing meristems. GFP
fluorescence can be seen from 4 days after Agrobacterium inoculation, depending on the expression
pattern. This ensures very rapid and obvious indication of the efficiency of each transformation
experiment. We will use a microplate based format for the growth and tracking of individual
transformants. We routinely use digital imaging techniques for archive and database construction,
and this information can be easily shared with colleagues at Ceres.
The main goal of this screen is to rapidly generate lines that possess highly specific patterns of
extensin-GFP expression for use as markers and for cell sorting experiments. Ideally, we wish to
collect lines where expression is limited or is absent from one or a few cell types (e.g. Fig 2). A
major advantage of screening primary transformants is that lines with no or broad expression
patterns can be discarded or pooled, and more interesting lines can be identified immediately. By
concentrating our initial efforts in this way, we expect to screen at least 12,000 transformed
lines The alternative (perhaps complementary) approach would be to generate transgenic lines using
an Agrobacterium -floral infiltration based approach. However, this would require much more
glasshouse space than we have available, and would not allow rapid prescreening for extensin-GFP
expression in primary transformants. I expect that the first batches of characterised seed could be
sent to Ceres within 6 months of starting the screen.
I am seeking funds to support two postdoctoral workers and one research technician to support this
screen. The screen will require a large amount of media preparation, basic microbial and plant
tissue growth and glasshouse planting and seed collection, and the efforts of a full time
technician will be required to support the work. The postdoctoral workers will be responsible for
the generation, screening, documentation and amplification of the extensin-GFP expressing lines.
The workers will have access to three confocal microscope systems and computers for 3D
reconstruction and detailed description of interesting lines. However, I have requested funds for a
Leica MZ FLIII epifluorescence stereomicroscope and computer for rapid screening and documentation
of transgenic plantlets. Our current system, based on an aging inverted epifluorescence microscope
is already over taxed. In addition, I have included a request for an additional
computer with CD-R writer to allow storage and physical segregation of Ceres-related data. The
consumables budget includes the cost of a large amount of plasticware, media, computer and
microscopy items and glasshouse supplies, including ara-cons for seed collection.
| Page 8 |
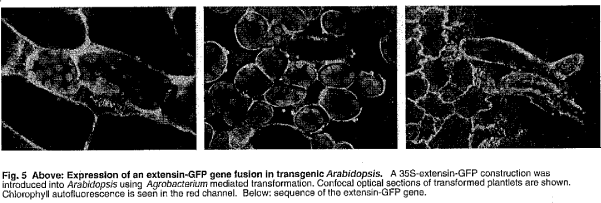
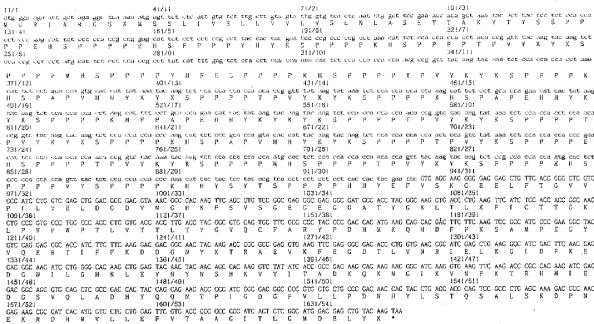
3. Screen for embryonic expression patterns.
The primary screen (described above) will result in the generation of over 12,000 transgenic
plantlets, which will be screened for specific patterns of extensin-GFP gene expression. On the
basis of our first GAL4-GFP screen, I expect that over 1000 lines will show extensin-GFP expression
as plantlets, and 200-400 of these should maintain stable and limited patterns of expression as
transgenic lines. All or as many as possible that space allows of those plantlets that do not
express the marker will be grown for seed collection. Ideally, we would like to rescue as many of
these 12,000 primary transformants as possible, and generate independent transformed lines. Lines
which possess bright and specific embryo and floral expression patterns often show little GFP
expression as plantlets. Therefore a secondary screen is necessary for detection of these
interesting patterns.
We performed a secondary screen using pooled seed from our first GAL4-GFP experiments. This
involved the microscope dissection of flowers and siliques from hundreds of transgenic plants, and
video documentation of fluorescence within dissected embryos and floral parts. This was slow and
tedious but allowed us to obtain a useful additional collection of GAL4-GFP expressing lines.
However, use of the
| Page 9 |
cell-wall tethered GFP marker and recent technical developments promise radical improvements in
this type of secondary screen.
The GFP-extensin marker is resistant to various clearing techniques that normally result in
complete loss of the protein from tissues prepared for microscopy. For example, the cell wall bound
signal is retained after complete alcohol dehydration or glycerol infiltration. We are now
experimenting with various gentle clearing treatments that will allow deep optical sectioning and
detailed 3D analysis of extensin-GFP marked tissues. For example, embryos can be optically
sectioned within ovules in benzyl alcohol: benzyl benzoate cleared intact siliques (Fig. 6). In
this case the clearing agent induces autofluorescence which would provide a useful counterstain. We
are continuing to experiment with different histological techniques, and I anticipate that a
judicious choice of clearing agent (which doesn’t adversely affect GFP fluorescence) will allow the
direct and highly precise observation of GFP-extensin expression deep within developing tissues.
Clearing agents include benzyl benzoate and glycerol based formulations. Of course, effective
clearing of GFP labelled tissues will have profound implications for the speed and precision of a
screen for floral and embryo expression patterns.
With some part-time support from undergraduate labour, the large number of the non-expressing
plantlets could be transferred to soil and coaxed to set seed. Flowers and siliques corresponding
to a range of developmental stages could be excised from the individual plants, dehydrated, cleared
and scored for extension-GFP expression by epifluorescence and confocal microscopy. In particular,
it should be relatively straightforward to document specific patterns of expression within embryos
at different stages of development. Unfortunately, one major impediment to a large scale secondary
screen is the lack of high quality glasshouse space (suitable for transgenic plants) in the
Department of Plant Sciences, University of Cambridge. The Department has a number of ancient
glasshouses and space for growth rooms, that are suitable for upgrading or equipping, and I am
raising funds for this. I have included a request for partial support of this necessary upgrading.
4. Sorting cell types and screening for gene expression patterns.
There is a compelling motivation for the screens described above. Tissues from transgenic
Arabidopsis lines that contain specific patterns of extensin-GFP expression can be treated with
pectinase to liberate individual cell types. The fluorescent extensin-GFP tagged cell types may
then be incubated with anti-GFP antibody coated magnetic particles, and specifically fished out of
the population of cells. The isolated cells can be checked by fluorescence microscopy to ensure
purity. If the cells are gently fixed (e.g formaldehyde treated) immediately before isolation, then
mRNA extracts will reflect the original
| Page 10 |
state of the cells. Extracted mRNAs can then be used for the construction of specific cDNA
libraries or for scoring gene expression patterns using various PCR-based or DNA chip technologies.
The access to different cell types will only be limited by availability of an appropriate
extensin-GFP expressing line. Clearly, our aim will be to provide as near a complete collection of
highly localised patterns as possible.
The same fishing technique will be useful for studying the protein components of specific cell
types, for example using antibody assays or fluorescent 20 gel display techniques. If one uses
unfixed cells, it will be possible to assay biochemical activities. In addition, it may be possible
to use sequential selection for different epitopes, to isolate cellular subpopulations.
I anticipate that this part of the work will be carried out largely at Ceres. However, our own work
involves mapping the functional and positional relationships between cell types within the
developing Arabidopsis root meristem. The root meristem is a highly dynamic network of related
cells, and the accurate mapping of changing patterns of gene expression will need to be correlated
with the 3D arrangements of particular cellular domains. In my view, 3D visualisation techniques,
live imaging of gene expression and targeted misexpression will be essential adjuncts to this
effort. I think that we will be in a position to provide some of these extra elements. In return,
we recognise the need to understand the expression patterns of key regulators within the root
meristem -both during normal development, and after selective genetic perturbation. For example, we
are selectively misexpressing certain homeodomain proteins in the root meristem, finding
post-translational regulation, and attempting to derive constitutively active variants. Cell
sorting and DNA chip analysis of potential target genes would be an immense help in decoding
potential networks of interacting regulators.
Schedule of work.
We aim to generate and characterise more than 12,000 Arabidopsis HAPI -extensin-GFP enhancer trap
lines over 3 years. This will be the most extensive screen of its kind to date. We will use a
combination of transformation techniques to generate these lines. First, tissue culture based root
transformation methods (A) allow rapid testing of vector efficiency and direct selection of
fluorescent primary transformants in the early stages of a screen. Second, floral dip
transformation methods (B) allow simple scaling up of the process, with little requirement for
experienced personnel, allowing postdoctoral workers to concentrate on the analysis and
documentation of the lines. The unique properties of the extensin-GFP marker will aid these
screens.
| (i) | Individual primary transformants can be directly screened for expression during culture. This allows the use of rapid, high efficiency tissue culture methods, and minimises the need for amplification of subsequent generations by large scale glasshouse plantings. (Previous large scale screens, which used Agrobacterium infiltration and the XXX xxxxxx, have involved teams of up to a dozen workers and hundreds of square metres of glasshouse space over many years, simply to generate the transformed material). | |
| (ii) | Cell-specific expression patterns can be immediately identified. Extensin-GFP provides a bright, insoluble fluor and tissue-specific expression can be precisely visualised and documented in live regenerated plantlets, and cleared floral and embryonic tissues, using epifluorescence and confocal microscopy. |
Such an immediate screen allows the rapid establishment of Arabidopsis lines with
highly selective patterns of HAPI -extensin-GFP expression, within a single generation in the case
of tissue culture based
| Page 11 |
transformation. Production of the Arabidopsis lines will involve (1) a series of
transformation experiments, (2) microscopic imaging, documentation and database construction, and
(3) amplification of selected lines. Two postdoctoral workers and a technician will be employed in
the generation and cataloguing of the lines.
(A). Tissue culture tranformation method
| Suface sterilization of Arabidopsis seed (20 mg, ~1000 seed) | ||
| addition to liquid media | ||
2 weeks
|
Germination and growth of wild-type seedlings | |
| addition of 2,4 D to liquid media | ||
3 days
|
Callus induction & root harvesting | |
| inoculation and transfer to CIM agar media in Petri dishes | ||
2 days
|
Agrobacterium cocultivation (~10,000 explants) | |
| transfer to selective SIM agar media | ||
4 weeks
|
Shoot induction (up to 1500 regenerated transformants) | |
| transfer to RIM agar media in 24-well dishes | ||
2 weeks
|
Root induction (60 dishes) | |
1 day
|
Screen 1: root and shoot expression of extensin-GFP; document patterns by video fluorescence microscopy transfer to agar media in magenta boxes | |
4 weeks
|
Grow plantlets to maturity (1500 lines) | |
4 days
|
Screen 2: floral, ovule and embryonic expression of extensin-GFP. Collect flowers and siliques in (60) 24-well dishes, dehydrate, clear and document patterns by video fluorescence microscopy | |
2 weeks
|
Dry plants; collect and archive the seed. |
(B). Floral dip tranformation method
| Planting of seed in glasshouse. | ||
4-6 weeks
|
Infiltrate emerging floral bolts with Agrobacterium culture containing the mHAP1-extensinGFP enhancer trap vector. | |
4 weeks
|
Harvest transformed seed. | |
2 weeks
|
Dry seed | |
| Surface sterilise seed and transfer to petri dishes containing selective media | ||
1 week
|
Score seedlings for presence of antibiotic resistance marker | |
1 day
|
Screen 1: root and shoot expression of extensin-GFP; document patterns by video fluorescence microscopy | |
| transfer transformed seedlings to soil | ||
4 weeks
|
Grow plantlets to maturity |
| Page 12 |
4 days
|
Screen 2: floral, ovule and embryonic expression of extensin-GFP. Collect flowers and siliques in 24-well dishes, dehydrate, clear and document patterns by video fluorescence microscopy. | |
2 weeks
|
Dry plants; collect and archive the seed. |
1. Generation of enhancer trap lines.
The time course of a typical transformation experiment is shown above. In this example, a
single worker would expect to produce around 1,500 transformants in 4 months, with some technical
support for the preparation of sterile media and growth vessels. There is considerable scope for
increasing the scale of individual transformation experiments, depending on coordination and
enthusiasm of those involved, or the recruitment of additional undergraduate labour. The
application includes funds for a technician who will be required to prepare considerable quantities
of plant tissue culture media.
2. Documentation of gene expression patterns.
With each transformation experiment, there will be a need to document all expression patterns.
About 15-25% of all lines will show some form of extension-GFP expression, based on our experience
with the GAL4-GFP screen. This documentation of the primary transformants is crucial, and we have
designed schemes to streamline the process as much as possible, using digital imaging and database
techniques. In addition, those lines which possess highly specific, bright and stable expression of
the GFP marker will be examined in detail, using confocal 3D imaging techniques to precisely map
expression patterns.
Each cycle of transformation, screening and documentation of the lines and seed collection will
xxxx 0 months. It will be feasible to generate 2,500 — 3,500 transgenic Arabidopsis plants over a
6 month period, with the support of 2 post-doctoral workers and a technician. This will allow the
screening of 12,000 individual transformants over the 3 year time period of the grant.
Projected timetable
Cambridge
| 6 months | 12 months | 18 months | 24 months | 30 months | 36 months | |||||||||||||||||||
Vector optimization |
||||||||||||||||||||||||
Plant transformation |
||||||||||||||||||||||||
Confocal 3D imaging |
||||||||||||||||||||||||
Database construction |
||||||||||||||||||||||||
Number of plantlets |
1,000 | 3,000 | 6,000 | 9,000 | 12,000 | |||||||||||||||||||
Establishment of lines |
1,000 | 3,000 | 6,000 | 9,000 | 12,000 | |||||||||||||||||||
Ceres
| 6 months | 12 months | 18 months | 24 months | 30 months | 36 months | |||||||||||||||||||
Seed from Cambridge |
||||||||||||||||||||||||
Growth of lines |
||||||||||||||||||||||||
Cell isolation and
microarray
screening |
||||||||||||||||||||||||
| Page 13 |
Funding Request
| Year 1 | Year 2 | Year 3 | total | |||||||||||||
Xx. Xxxxx Xxxxx |
£ | 28,099 | £ | 30,350 | £ | 32,722 | £ | 91,171 | ||||||||
Post-doc 2 |
£ | 26,922 | £ | 29,083 | £ | 31,412 | £ | 87,417 | ||||||||
Technician |
£ | 18,000 | £ | 19,205 | £ | 20,076 | £ | 57,281 | ||||||||
Overhead costs1 |
£ | 51,115 | £ | 55,047 | £ | 58,947 | £ | 165,109 | ||||||||
Glasshouse
services2 |
£ | 10,000 | £ | 10,000 | £ | 10,000 | £ | 30,000 | ||||||||
Consumables |
£ | 31,000 | £ | 32,000 | £ | 33,000 | £ | 96,000 | ||||||||
Equipment 3 |
£ | 24,000 | £ | 24,000 | ||||||||||||
Glasshouse upgrade |
£ | 20,000 | £ | 20,000 | ||||||||||||
Travel |
£ | 1,500 | £ | 1,500 | £ | 1,500 | £ | 4,500 | ||||||||
Total |
£ | 210,636 | £ | 177,185 | £ | 187,657 | £ | 575,478 | ||||||||
| 1 | Overhead costs are levied by the University of Cambridge at 70% of the salary costs. Salaries are calculated according to the University Salary Scale, and include a salary calculated for Xx. Xxxxx Xxxxx who I am supporting at present; a postdoctoral worker of 30 years of age at start, and a technician starting at grade T4/05, with cost-of-living increments calculated at 3.5% per annum. Salaries include N.I. and U.S.S. subscriptions. | |
| 2 | Glasshouse services includes funding for part-time undergraduate labour. | |
| 3 | Equipment for primary screen. |
Leica MZ FLIII epifluorescence stereomicroscope with
integrating digital camera and image capture computer |
£ | 20,000 | ||
Apple Macintosh G3 with additional 18 GB hard disk and CD-R writer
and monitor. |
£ | 4,000 |
Exhibit B
Research Budget
Exhibit C
Material Transfer Agreements TYPE A and TYPE B
[For Purpose of Article 7.7]
UNIVERSITY OF CAMBRIDGE
MATERIALS TRANSFER AGREEMENT TYPE A
MATERIALS TRANSFER AGREEMENT TYPE A
Biological Materials to which this Agreement applies:
and any related confidential information (“Information”) and/or biological materials supplied in
connection therewith by the University of Cambridge (U of C), and any products that are replicated
or obtained through use therefrom by Recipient (the “Biological Materials”).
We are pleased to provide Recipient with the Biological Materials and related Information from the
laboratory of Xx. Xxxxx Xxxxxxxx (“Scientist”) of U of C, subject to the following terms:
| 1. | Recipient agrees that the Biological Materials shall be used solely for noncommercial research purposes to: |
hereinafter called “Research”. This work is to be conducted in the Recipient Scientist’s
Laboratories at the Institution identified below. The Biological Materials and related Information
will not be used for testing in or treatment of humans, and shall not be used, directly or
indirectly, for commercial purposes or on behalf of or to the benefit of any commercial entity or
business.
2. The Biological Materials will not be distributed further to third parties for any purpose. In
addition, Recipient shall obtain acceptance of the terms of this Agreement of all persons under its
direct control and supervision who have access to the Biological Materials and Information.
It is further understood by Recipient that any and all proprietary rights, including but not
limited to patent rights, trademarks and proprietary rights, in and to the Biological Materials and
Information shall be and remain in U of C, subject to the rights granted herein and subject to any
rights that Ceres, Inc. may have.
3. Nothing in this Agreement grants any rights under any patents or in any know-how of U of C nor
any rights to use the Biological Materials and related Information or any product or process
related thereto or obtained through use therefrom for profit-making or commercial purposes such as,
but not limited to, production, sale, screening or design.
4. U of C makes no representation that the use of the Biological Materials will not infringe any
patent or other proprietary right of any third party. The Biological Materials are provided only
to
Recipient and only for research purposes. Such materials are provided without warranty of
merchantability or fitness for a particular purpose or any other warranty, express or implied.
It is understood that U of C and its employees and agents have no liability in connection with such
Biological Materials or their use.
5. In no event shall U of C be liable for any use of the Biological Materials and related
Information by the Recipient. Ceres shall not be liable for the Biological Materials and the
related Information, or their use by Recipient.
6. This Agreement will terminate in twenty (20) years after its signature date.
7. This Agreement may not be assigned by Recipient without the prior written consent of the U of C.
8. This Agreement sets forth the entire agreement and understanding between the parties and cannot
be changed or amended except by written agreement executed by both parties.
9. This agreement shall be construed in accordance with English law.
The authorized signatures below verify agreement between the parties.
| FOR THE UNIVERSITY of CAMBRIDGE | ||||||||
By
|
: | DATE: | ||||||
| Xxxxxxx X Xxxxxxxx Ph.D. | ||||||||
| Director | ||||||||
| Xxxxxxx Industrial Liaison Office | ||||||||
| University of Cambridge | ||||||||
| 00 Xxxxxxxxxxx Xxxxxx, Xxxxxxxxx XX0 0XX | ||||||||
By
|
: | DATE: | ||||||
| SCIENTIST’s SIGNATURE | ||||||||
| FOR THE RECIPIENT: | ||||||||
| [Insert Institution Name and Address] | ||||||||
By
|
: | DATE: | ||||||
| Authorized Institutional Representative: | ||||||||
| Position: | ||||||||
By
|
: | DATE: | ||||||
| RECIPIENT SCIENTIST’s SIGNATURE |
[For Purpose of Article 7.8]
UNIVERSITY OF CAMBRIDGE
MATERIALS TRANSFER AGREEMENT TYPE B
Biological Materials to which this Agreement applies:
and any related confidential information (“Information”) and/or biological materials supplied in
connection therewith by the University of Cambridge (U of C), and any products that are replicated
or obtained through use therefrom by Recipient (the “Biological Materials”).
We are pleased to provide Recipient with the Biological Materials and related Information from the
laboratory of Xx. Xxxxx Xxxxxxxx (“Scientist”) of U of C, subject to the following terms:
| 1. | Recipient agrees that the Biological Materials shall be used solely for noncommercial research purposes to: |
hereinafter called “Research”. This work is to be conducted in the Recipient Scientist’s
Laboratories at the Institution identified below. The Biological Materials and related Information
will not be used for testing in or treatment of humans, and shall not be used, directly or
indirectly, for commercial purposes or on behalf of or to the benefit of any commercial entity or
business.
2. The Biological Materials will not be distributed further to third parties for any purpose. In
addition, Recipient shall obtain acceptance of the terms of this Agreement of all persons under its
direct control and supervision who have access to the Biological Materials and Information.
It is further understood by Recipient that any and all proprietary rights, including but not
limited to patent rights, trademarks and proprietary rights, in and to the Biological Materials and
Information shall be and remain in U of C, subject to the rights granted herein and subject to any
rights that Ceres, Inc. may have.
3. Nothing in this Agreement grants any rights under any patents or in any know-how of U of C nor
any rights to use the Biological Materials and related Information or any product or process
related thereto or obtained through use therefrom for profit-making or commercial purposes such as,
but not limited to, production, sale, screening or design.
4. U of C makes no representation that the use of the Biological Materials will not infringe any
patent or other proprietary right of any third party. The Biological Materials are provided only
to Recipient and only for research purposes. Such materials are provided without warranty of
merchantability or fitness for a particular purpose or any other warranty, express or implied. It
is understood that U of C and its employees and agents have no liability in connection with such
Biological Materials or their use.
5. Thirty (30) days before their submission, Recipient shall provide to U of C copies of all
posters, abstracts and publication manuscripts describing data obtained under the Research. U of
C will promptly disclose these posters, abstracts and publication manuscripts to Ceres in
confidence for the purposes of Ceres initiating a dialog with the Recipient to explore
opportunities for collaboration.
6. Confidentiality. Recipient agrees to hold in strictest confidence the Biological Materials, the
nature of the Biological Materials and Information being used except for information which:
a. is included in publications of results of the Research which are made in accordance
with the term of this Agreement; or
b. was lawfully in Recipient’s possession or control prior to the date of disclosure;
or
c. was in the public domain or enters into the public domain through no improper act on
Recipient’s part or on the part of any of Recipient’s employees; or
d. is rightfully given to Recipient from sources independent of U of C; or
e. was independently developed by employees of the Recipient without knowledge of the
Information provided by U of C, as demonstrated with written records; or
f. must be disclosed for minimum lawful compliance with court orders, regulations and
statutes.
7. In no event shall U of C be liable for any use of the Biological Materials and related
Information by the Recipient. Ceres shall not be liable for the Biological Materials and the
related Information, or their use by Recipient.
8. This Agreement will terminate when the Biological Materials become generally available to third
parties through an M.T.A. Type A (enclosed herewith as Exhibit), and such M.T. A. Type A will
substitute the present M.T.A. Type B.
9. This Agreement may not be assigned by Recipient without the prior written consent of the U of C.
10. This Agreement sets forth the entire agreement and understanding between the parties and cannot
be changed or amended except by written agreement executed by both parties.
11. This agreement shall be construed in accordance with English law.
The authorized signatures below verify agreement between the parties.
| FOR THE UNIVERSITY of CAMBRIDGE | ||||||||
By
|
: | DATE: | ||||||
| Xxxxxxx X Xxxxxxxx Ph.D. | ||||||||
| Director | ||||||||
| Xxxxxxx Industrial Liaison Office | ||||||||
| University of Cambridge | ||||||||
| 00 Xxxxxxxxxxx Xxxxxx, Xxxxxxxxx XX0 0XX | ||||||||
By
|
: | DATE: | ||||||
| SCIENTIST’s SIGNATURE | ||||||||
| FOR THE RECIPIENT: | ||||||||
| [Insert Institution Name and Address] | ||||||||
By
|
: | DATE: | ||||||
| Authorized Institutional Representative: | ||||||||
| Position: | ||||||||
By
|
: | DATE: | ||||||
| RECIPIENT SCIENTIST’s SIGNATURE | ||||||||
Exhibit D
List of Patent Rights related to University Background Technology
Exhibit E
List of Collaborators of Xx. Xxxxxxxx
Xx. Xxxxxxxx Xxxxxx
RCAP/INRA
Ecole Normale Superior Lyon
46 Allee d’Italie
Lyon
France
RCAP/INRA
Ecole Normale Superior Lyon
46 Allee d’Italie
Lyon
France
Xx. Xxxxx Xxxxxxxx
Department of Biology
University of York
York
United Kingdom
Department of Biology
University of York
York
United Kingdom
Dr Xxxx Xxxx
Department of Plant Sciences
University of Cambridge
Xxxxxxx Xxxxxx
Xxxxxxxxx XX0 0XX
Xxxxxx Xxxxxxx
Department of Plant Sciences
University of Cambridge
Xxxxxxx Xxxxxx
Xxxxxxxxx XX0 0XX
Xxxxxx Xxxxxxx
Xx. Xxxx Xxxxxx
Department of Plant Sciences
University of Cambridge
Xxxxxxx Xxxxxx
Xxxxxxxxx XX0 0XX
Xxxxxx Xxxxxxx
Department of Plant Sciences
University of Cambridge
Xxxxxxx Xxxxxx
Xxxxxxxxx XX0 0XX
Xxxxxx Xxxxxxx
Xx. Xxxxx Xxxxxxx
Department of Biology
University of Pennsylvania
000 X. Xxxxxxxxxx Xxxxxx
Xxxxxxxxxxxx, XX 00000-0000
XXX
Department of Biology
University of Pennsylvania
000 X. Xxxxxxxxxx Xxxxxx
Xxxxxxxxxxxx, XX 00000-0000
XXX
Draft: October 17, 2001
Stricly Confidential
Amendment I
Effective as of October 1, 2001 (the Amendment I Effective Date)
to the Sponsored Research Agreement between The Chancellor, Masters and Scholars of the University
of Cambridge (“University”) and Ceres, Inc. (“Ceres”) of June 1, 2000 (the “Agreement”).
WHEREAS certain Technology was developed at Ceres that may be useful for the Research Project;
WHEREAS the parties wish to expand the Research Project in order to include certain activities
based on such Technology;
NOW THERFORE, the Parties agree as follows:
| 1. | Section 1.2. of the Agreement — definition of “Background Technology” shall be amended by adding at the end of the present text: |
“. . .Exhibit D. In addition, Ceres Background Technology shall include
Technologies relating to recombinant transmembrane proteins as defined in
Exhibit I to Amendment I to this Agreement, developed prior to the Amendment
I Effective Date.”
| 2. | A new paragraph is inserted between the first and the second paragraph of Section 4 in the Chapter “Proposal” in Exhibit A to the Agreement, the text of which paragraph is set forth in Exhibit I to this Amendment I. This addition to the Research Project is referred to hereinafter, when distinguished from the remainder of the Research Project, as Addition to Research Project. |
| 3. | A new Section is added to the Chapter Funding request in Exhibit A to the Agreement, the text of which is set forth in Exhibit I to this Amendment I. |
| 4. | For the avoidance of doubt, any results arising from work performed by University with the Ceres Background Technology referred to in Exhibit I to this Amendment I shall be deemed to be Project Technology. |
Page 1 of 4
Draft: October 17, 2001
Stricly Confidential
| 5. | In Section 7.6 on Publication and Patentability, the following clauses shall be added at the end: |
| 7.6.1. | Notwithstanding the above, any student of the University working on the Addition to the Research Project (Student) may include some or all of the results acquired during the work on the Addition to the Research Project in a thesis submitted for a degree of the University. The thesis shall be examined by examiners appointed by the University and a successful thesis deposited in the University library in accordance with University Regulations. The Student shall, on request of Ceres, request that access to the thesis be restricted initially for two (2) years and then for a period of up to five (5) years, for one year at a time. This request by Ceres shall not be unreasonably denied by the University. | ||
| 7.6.2 | The examiners of the thesis will examine the thesis in confidence according to University regulations, but Ceres may, at its discretion, request that the examiners agree in writing to be bound by the terms of confidentiality as set out in Sections 7.4 and 7.6 of the Agreement. |
| 6. | For the remainder, the Agreement shall be unchanged and continue in full force and effect, and this Amendment shall constitute an integral part thereof. |
IN WITNESS WHEREOF the respective Parties hereto have executed this Amendment I by their duly
authorized officers as of the Amendment I Effective Date.
| THE UNIVERSITY OF CAMBRIDGE | CERES, INC. | |||||
By:
|
By: | /s/ Xxxx Xxxxx | ||||
| Xxxx Xxxxx | ||||||
Title:
|
Director of Research Services | Title: | Chief Operating Officer | |||
By:
|
/s/ Xxxxx Xxxxxxxx | By: | /s/ Xxxxxxx Xxxxxxx | |||
| Xxxxx Xxxxxxxx | Xxxxxxx Xxxxxxx, CBE, FRS | |||||
Title:
|
Lecturer | Title: | Chief Scientific Officer | |||
Page 2 of 4
Draft: October 17, 2001
Stricly Confidential
Exhibit I
to Amendment I to the Sponsored Research Agreement
| 1. | Certain Ceres Background Technology: Recombinant transmembrane proteins. | |
| There are a range of other opportunities for cell sorting in addition to using cell wall-insolubilized GFP-extensin. Protoplasts can be isolated from a variety of tissues in Arabidopsis and many other plant species, and it might be possible to use ligands exposed at and attached to the surface of the plasma membrane for sorting. One possibilty is to explore GFP-tagged proteins arising from random gene fusion that localize to the plasma membrane, such as the PIP fusion from the Carnegie Institute. Another possibility is to engineer transmembrane proteins such as receptor kinases or other proteins with secretion signals and hydrophobic domains such that they carry a fluorescing protein such as GFP to the cytosolic face of the membrane, and a ligand such as an antigen to the extracellular face. Protoplasts prepared from tissues carrying a bifunctional protein such as this could allow sorting of protoplasts visualized by fluorescence. A recombinant receptor kinase and a recombinant permease have already been assembled at Ceres, and these could form the basis of these experiments. | ||
| 2. | Proposal: New text to be included as paragraph 2 of Section 4 | |
| Two recombinant transmembrane proteins — a receptor kinase and a permease - have also been generated at Ceres, and both have been introduced into plants. To explore the potential of these proteins for protoplast sorting, we will use confocal microscopy to see if these recombinant proteins carry GFP to the inner face of the plasma membane. If either of them do, we will use anti-FLAG antibody to see if they also deliver FLAG epitopes to the other face. This can be done by immunofluorescence on sectioned material or protoplasting and addition of anti-FLAG antibody carrying small beads. Proteins that provide GFP and FLAG to opposite surfaces of the plasma membranes will be expressed in Hap1-activation lines, the cells and tissue so identified being targets for confocal imaging and cell sorting. Fluorescing protoplasts can be sorted from nonfluorescing protoplasts by applying magnetic beads to the extracellular ligands. These magnetic beads will be applied as antibody conjugates or other kinds of conjugates. Scientists at Ceres have direct experience with magnetic separation procedures and can help with the sorting. | ||
| In case the Ceres Background Technology referred to in Exhibit I to Amendment I to the Agreement does not work correctly, a set of transmembrane proteins will be engineered such that GFPs are added to the exposed cytosolic domains and FLAG, histidine or c-myc tags added to the extracellular domains. This can be in |
Page 3 of 4
Draft: October 17, 2001
Stricly Confidential
| a systematic way, so that all the receptor kinases, for example, in the Arabidopsis genome are processed for cell sorting applications. When expressed in plants, | ||
| these recombinant proteins will also allow visualization of cells that are also marked by extracellular tags. | ||
| 3. | Funding request: addition |
| Year 1 | ||||||||
| October 1, 2001 to | ||||||||
| September 30, 2002 | Total | |||||||
Graduate Student |
£ 28,000 | £ | 28,000 | |||||
Page 4 of 4
Xxxx Xxxxx Xxxxxxxx
Contracts Manager
Contracts Manager
Xxxxxxx Xxxxxxx |
 |
|
Chief Scientific Officer
|
||
Ceres Inc |
||
0000 Xxxxxx Xxxxxx Xxxx
|
Research Services Division | |
Xxxxxx |
||
XX 00000 |
||
XXX |
| 22 May 2003 | When replying please quote: RG31274 |
Dear Xx Xxxxxxx,
Studentship for Xxxx Xxxxx — Continuation to PhD
‘Decoding gene expression and plant cell fate’
‘Decoding gene expression and plant cell fate’
I am writing with reference to your correspondence with Xxxx Xxxxx and Xxxxxxxxx Xxxxxxxx of
October 2002, in which you state that you are willing to provide $40,000 funding per year for 2003
and 2004, for the continuation of Xxxx Xxxxx’x research.
In order that this additional funding can be activated on our system, I would be grateful if you
could confirm that you are willing for the end date of the original contract to be extended in
order to cover the renewed time period for Xxxx Xxxxx’x funding. If this acceptable to you please
could you sign and return the duplicate of this letter, which shall be taken as your agreement that
the duration of the Sponsored Research Agreement shall be extended to 31st December
2004.
May I take this opportunity to thank you for sponsoring this research and for the additional
funding for Xxxx Xxxxx.
If you have any questions relating to this, please do not hesitate to contact me.
Best Regards,
| /s/ Xxxxx Xxxxxxxx | ||||
| Xxxxx Xxxxxxxx | ||||
I agree to extension of the Sponsored Research Agreement between The University of Cambridge
and Ceres, Inc. as outlined above.
Signed: /s/ Xxxxxxx Xxxxxxx
|
Date: June 4, 2003 |
Title: CSO
| 00 Xxxx Xxxx Xxxxxxxxx |
||
| XX0 0XX | ||
| Telephone: 00000 000000 | ||
| Fax: 00000 000000 | ||
| E-mail: xxxxx.xxxxxxxx@xxx.xxx.xx.xx |

