Manufacture and Supply Agreement
In this document, “[***]” indicates that confidential materials have been redacted from this document and filed separately with the Securities and Exchange Commission.
Exhibit 10.7
This Manufacture and Supply Agreement (the “Agreement”) is entered into and made effective as of this 1st day of January 2018 (the “Effective Date”), by and between:
1. | Cayman Chemical Company, Incorporated, a Colorado Corporation licensed to do business in Michigan, whose registered office is located at 0000 X. Xxxxxxxxx Xxxx, Xxx Xxxxx, Xxxxxxxx, 00000, XXX (hereinafter referred to as “Cayman”); and |
2. | Aerie Distribution, Incorporated, a corporation organized under the laws of Delaware, USA, and a wholly-owned subsidiary of Aerie Pharmaceuticals, Incorporated, having a place of business at 0000 Xxxxxxx Xxxx., Xxxxx 000X, Xxxxxx, XX 00000, XXX (hereinafter referred to as “Aerie”), |
each a “Party” and together the “Parties”.
RECITALS
WHEREAS, Cayman is a worldwide manufacturer of active pharmaceutical ingredients (“APIs”); and
WHEREAS, Aerie and its Affiliates desire to produce formulated drugs for various markets, utilizing APIs manufactured by Cayman; and
NOW, THEREFORE, in consideration of the mutual promises and covenants herein contained, the Parties hereto agree as follows:
ARTICLE 1.0 | DEFINITIONS |
1.1. | “Affiliate” shall mean any corporation or non-corporate business entity which controls, is controlled by, or is under common control with a party to this Agreement. A corporation or non-corporate business entity shall be regarded as in control of another corporation if it owns, or directly or indirectly controls, at least fifty (50%) percent of the voting stock of the other corporation, or: (i) in the absence of the ownership of at least fifty (50%) percent of the voting stock of a corporation; or (ii) in the case of a non-corporate business entity, or non-profit corporation if it possesses, directly or indirectly, the power to direct or cause the direction of the management and policies of such corporation or non-corporate business entity, as applicable. A corporation or non-corporate business entity shall also be regarded as in control of another corporation if in any country where local law will not permit foreign equity participation of a majority, ownership, or control, directly or indirectly, of the maximum percentage of such outstanding stock or voting rights permitted by local law. |
1.2. | “API” shall mean the Active Pharmaceutical Ingredient manufactured as identified in Exhibit A. |
1.3. | “Certificate of Analysis (COA)” is a listing of all results for tests conducted on samples of a batch of API compared to the Specifications defined by Aerie, listed in regulatory applications, and applicable compendia. |
1.4. | “CGMP” shall mean the current Good Manufacturing Practices promulgated by the FDA, ICH, European Commission (Eudralex - Volume 4, Parts II and III as applicable to non-sterile active substances), Health Canada, and PMDA as amended from time to time. CGMP shall also include good manufacturing practice regulations promulgated by a Regulatory Authority in a Territory added to this Agreement after the Effective Date of this Agreement, solely to the extent Aerie or its designee has provided access to written copies of such regulations to Cayman prior to Cayman’s manufacturing of Product under this Agreement. Copies of all regulations shall be in the English language. |
1
1.5. | “Date of Manufacture” shall be as notated in the applicable step of the executed master batch record for the batch and as recorded on the COA. |
1.6. | “Facility” or “Facilities” shall mean Cayman’s US facility located at 0000 X. Xxxxxxxxx Xxxx, Xxx Xxxxx, XX, 00000, XXX. |
1.7. | “FDA” shall mean the United States Food and Drug Administration, and any successor entity thereto. |
1.8. | “Gross Negligence” shall mean conduct that evinces a reckless disregard for or indifference to the rights of others, risk with respect to loss of Product, or lack of adherence to CGMP or approved procedures (e.g., MBRs or approved standard operating procedures). |
1.8.1. | For the avoidance of doubt the following examples are considered to be Gross Negligence: (i) lack of proper care in handling materials, such as the placement of material in an unstable location resulting in a spill and subsequent loss and (ii) not following the master batch record instructions, such as the addition of incorrect material during execution of a MBR resulting in unrecoverable loss. |
1.9. | “Health Canada” shall mean the Therapeutic Products Directorate of Canada, and any successor entity thereto. |
1.10. | “ICH” shall mean the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use, and any successor entity thereto. |
1.11. | “Intellectual Property (IP)” shall mean any patents, utility models, trademarks, service marks, rights in designs, copyrights, rights in databases, and rights in know-how (whether or not any of these is registered or capable of registration and including applications for registration of any such thing), and all other similar rights or forms of protection of a similar nature or having equivalent or similar effect to any of these which may subsist anywhere in the world. |
1.12. | “Intermediate” shall mean an Intermediate manufactured during the process of manufacturing the Product and shall be produced according to CGMP and manufactured using an appropriately validated process. |
1.13. | “Latent Defect” shall mean a defect that was not identifiable or knowable using reasonable means of analysis. Latent Defect does not include inherent properties of the Product. |
1.14. | “Marketing Application” shall mean an application for marketing authorization which has not yet been approved by the FDA or other Regulatory Authority, including without limitation, FDA Investigational New Drug Application (IND), FDA New Drug Application (NDA), FDA Abbreviated New Drug Application (ANDA), and other similar marketing applications and/or supplements to approved applications promulgated by Regulatory Authorities in any jurisdiction. |
1.15. | “Marketing Authorizations” shall mean any approved application for marketing authorization, including without limitation, IND, NDA, ANDA (as such terms are used by the FDA), and other similar marketing authorizations promulgated by Regulatory Authorities in any jurisdiction. |
1.16. | “MBR” shall mean a master batch record for manufacturing and packaging of the Product that is adequate to comply with the applicable requirements and standards of such regulatory agencies and health authorities to which Aerie shall submit their US NDA or comparable application in other jurisdictions. |
1.17. | “Minimum Purchase Requirements” shall mean the minimum quantity of Product purchased by Aerie by Year as indicated in Exhibit B. |
1.18. | “PMDA”“ shall mean the Japanese Pharmaceuticals and Medical Devices Agency, and any successor entity thereto. |
2
1.19. | “Ph Eur” shall mean European Pharmacopoeia. |
1.20. | “Product” shall mean the API identified in Exhibit A and produced according to CGMP and manufactured using an appropriately validated process. |
1.21. | “Quality and Technical Agreement (QTA)” shall mean the current quality and technical agreement with an effective date of 07 Jan 2016 and any amendments or successive agreements thereto. |
1.22. | “Regulatory Authority” shall mean the FDA and any other applicable authority within a Territory involved in regulating any aspect of the development, manufacture, market approval, sale, distribution, packaging, or use of the Aerie APIs or drug products formulated therewith. |
1.23. | “Specifications” shall mean the particulars as to composition, quality, and other characteristics for the Product as referenced in Exhibit A hereto, as may be amended from time to time by mutual agreement of the Parties. |
1.24. | “Territories” shall mean the United States of America, Canada, Europe, Japan, and any other region or country which Aerie may propose in writing to add to this Agreement with Cayman’s written consent, such consent not to be unreasonably withheld. |
1.25. | “USP” shall mean United States Pharmacopoeia. |
1.26. | “Willful Misconduct” shall mean when a person intentionally acts or fails to act knowing that (his, her) conduct will probably result in injury, damage, or loss of starting material, Intermediate, or Product. |
1.26.1. | For the avoidance of doubt the following examples are considered to be Willful Misconduct: (i) intentional addition of incorrect material and (ii) failure to clean a spill resulting in a slip and fall and subsequent loss. |
1.27. | “Year” shall mean calendar year commencing on January 1 and ending December 31. |
ARTICLE 2.0 SUPPLY
2.1 | Supply. Cayman shall supply to Aerie quantities of the Product ordered by Aerie within the Territory from time to time in accordance with this Agreement and the forecasts provided in Section 2.3. Without limiting the foregoing, Cayman shall at all times maintain facilities to manufacture the Product as described in the MBR. |
2.1.1. | All Product shall be manufactured at and supplied from the Facility. |
2.1.2. | The Product shall be supplied in packages as described in Section 2.7 containing the amounts as designated on Aerie’s order. |
2.1.3. | Quantities of Product to fulfill an order should come from the most recent single batch of Product. If multiple batches are required to fulfill an order amount, the Date of the Manufacture for the applicable batches cannot be separated by more than ninety (90) days. |
2.2. | Orders. Quantities of the Product will be supplied by Cayman pursuant to purchase orders submitted by Aerie to Cayman as stipulated in Section 2.3. |
2.2.1 | The quantities ordered shall be set according to the binding forecasts as indicated in Section 2.3.1 and shall fulfill the Minimum Purchase Requirements (Exhibit B). |
2.2.2. | Cayman agrees to accept Aerie’s purchase orders for the Product, provided that the purchase order is in accordance with the Binding Forecast and other stipulations of this |
3
Agreement and provides for a lead time of not less than one hundred twenty (120) days with planned order fulfillment in the month of December.
2.2.2.1. | Cayman’s obligation to supply within this lead time period shall be limited to those quantities included in the binding forecast. |
2.2.2.2. | Cayman shall not be penalized for order fulfillment in advance of the date requested in the purchase order. |
2.2.3. | Aerie agrees to accept the total quantity of the Product manufactured in a given single batch, provided that scale of the applicable manufacturing campaign was targeted based on the quantity in the purchase order based on the yield range for the step. |
2.3. | Forecasts. The non-binding and binding forecasts shall be updated by Aerie within five (5) business days of the first calendar day of each month. The total Binding Forecast and Non-Binding Forecast will be for an eighteen (18) month period. |
2.3.1. | Binding Forecasts. Beginning 01May2018, Aerie will provide to Cayman a binding six (6) month forecast starting from the end of the previous period. The binding six (6) month forecast will set forth desired fulfillment dates with quantities that Aerie will be obligated to buy according to the Minimum Purchase Requirements (Exhibit B) and Cayman will be obligated to supply. |
2.3.2. | Non-Binding Forecasts. Aerie will provide to Cayman a non-binding twelve (12) month forecast starting from the end of the period in the Binding Forecast. The non-binding twelve (12) month forecast will assist Cayman in planning and capacity allocation. |
2.3.3. | Aerie may request the acceleration of an order in advance of the fulfillment dates as specified in the Binding Forecast (Section 2.3.1) or the accepted purchase order; in this event Cayman shall use all commercially reasonable efforts to fulfill this request. If Cayman successfully meets the amended fulfillment date, a [***] premium shall be applied to the applicable Pricing in Exhibit B for the specific quantity ordered. |
2.4. | Form of Orders. Aerie’s orders shall be made pursuant to a written purchase order which is in a form acceptable to Cayman, and shall provide for shipment in accordance with reasonable fulfillment and delivery schedules specified in such purchase order, it being acknowledged and agreed that a fulfillment and delivery schedule specifying delivery [***] days or more from the date of the purchase order (or such shorter time as the Parties may reasonably agree in the circumstances) shall be considered reasonable. Purchase orders may be submitted by Aerie (or an Affiliate thereof) on behalf of itself or its Affiliates. Purchase orders shall include at minimum: |
2.4.1. | PO number |
2.4.2. | Quantity |
2.4.3. | Target Fulfillment Date |
Cayman shall provide a written order acknowledgement form within five (5) business days from receipt of Aerie’s purchase order confirming the quantities and fulfillment date for the order, including whether Cayman will be able to fill any amounts of such order in excess of the quantities that Cayman is obligated to supply pursuant to Section 2.1 above. This written order acknowledgement form will be regarded as a binding irrevocable commitment by Aerie and/or its Affiliates to purchase, and for Cayman to manufacture and supply, the relevant quantity of Product. No terms contained in any purchase order, order acknowledgment, or similar standardized form shall be construed
4
to amend or modify the terms of this Agreement and in the event of any conflict, this Agreement shall control unless expressly agreed in writing.
2.5 | Additional Quantities. Notwithstanding anything herein to the contrary, Cayman agrees to use all commercially reasonable efforts to supply any quantities ordered by Aerie in excess of such amounts as Aerie may forecast in accordance with Section 2.3 above, provided that any failure to do so shall not constitute a breach of this Agreement. |
2.6. | Price. The price to be paid by Aerie per kilogram (as rounded to the nearest hundredth of a kilogram) of the Product ordered by Aerie shall be based upon the quantities of the Product ordered and fulfilled by Cayman during a particular calendar year as described in Exhibit B. Payments shall be made according to the Payment Schedule in Exhibit B. |
2.7. | Packaging. Product shall be shipped to Aerie or their designee from Cayman’s Facility (i) according to the packaging as described in the Specifications and the MBR; (ii) in outer packing material sufficient to prevent breakage of bottles while in transport and maintain the required temperature range throughout the transport to Aerie or their designee; and (iii) with a packaging label(s) displaying all attributes as described in the MBR. |
2.7.1 | Additional Packaging operations documented in a Packaging Batch Record (PBR) and related scheduling and costs are outside the scope of this agreement. |
2.7.2. | A copy of a COA for the batch shall accompany such shipment along with all required shipping documentation based on final destination. |
2.8. | Storage. Cayman shall provide proper storage for raw materials, starting materials, intermediates, and Product. |
2.8.1. | Aerie will have Aerie Vendor(s)1 ship to Cayman advance inventory of starting materials for manufacturing of Product to the extent required to meet the quantities in the Binding Forecast and Non-Binding Forecast. |
2.8.2. | If Product is not shipped immediately from the Facility, Cayman will provide storage of Product up to the capacity of Aerie owned freezer(s) within the Facility (see Exhibit D). |
2.9. Stoppages.
2.9.1. | Aerie shall have the right to direct Cayman to stop work (a “Stoppage”); such fees described in Section 2.9.5 shall apply. |
2.9.2. | Cayman shall have the right to issue notice to Aerie that a Stoppage has occurred after [***] in the dedicated GMP Manufacturing Suite with the Aerie Purchased Equipment (per Section 2.16), if not resolved via issuance of a PO or resumption of production within [***], such fees described in Section 2.9.5 shall apply. |
2.9.3. | In the event Aerie issues a notice of Stoppage, Aerie will provide reasonable good faith estimate of the duration of the Stoppage as soon as is commercially reasonable. |
2.9.4. | For the avoidance of doubt, the effective date of the Stoppage is deemed to be the [***] after the day that work was actually stopped by Cayman in accordance with the instruction received from the applicable Party. |
1 | As defined in the QTA | |
5
2.9.5. | Fees. |
2.9.5.1. | For each month or fraction thereof in which Cayman’s Aerie-dedicated suite is subject to a Stoppage, Aerie will pay a fee (the “Stoppage Fee”) to Cayman at a rate of $[***]/month (annually adjustable for inflation based on the United States’ Producer Price Index (USPPI PCU325325)), provided that: |
2.9.5.1.1. | following a Stoppage Cayman shall use reasonable efforts to re-allocate capacity and resources to third parties and otherwise mitigate its losses, and Aerie shall pay the Stoppage Fee, or a portion thereof, in respect of losses that cannot be mitigated having exercised such efforts; and |
2.9.5.1.2. | if such mitigation requires re-allocation of the dedicated suite and Aerie Purchased Equipment (per Section 2.16), and such written permission is withheld, the full Stoppage Fee shall apply. |
2.9.5.1.3. | no Stoppage Fee will be payable in any Year in which the quantity of material in Aerie purchase orders accepted by Cayman for the applicable Year meets the Minimum Purchase Requirements. |
2.9.5.2. | The initial Stoppage Fee shall be invoiced upon receipt of a notice of Stoppage per Section 2.9.1 or 2.9.2 and payable per the terms in Section 2.14; subsequent monthly Stoppage payments are due on the first of the month subsequent to the initial Stoppage. |
2.9.6. | Restarts from Stoppages. |
2.9.6.1. | Timing. Upon receiving receipt of written notice to resume production (PO is considered an accepted form of notice) Cayman shall provide the date for the resumption of manufacturing suitable to allow for appropriate release of materials and manufacturing areas. |
2.9.6.2. | If in any Year Aerie makes Stoppage Fee payments, but goes on to place purchase orders that are accepted by Cayman thereby meeting the Minimum Purchase Requirements in that Year, all Stoppage Fee payments already paid will be reimbursed by Cayman or credited against future orders. |
2.9.7. | Stoppage from routine manufacture is permissible upon mutual and written agreement between both parties for the demonstration and/or validation of second generation process improvements at scale. No Stoppage Fee shall be applied during a Stoppage of mutual agreement for the demonstration and or validation of second generation process improvements. |
2.9.7.1. | Any costs associated with the demonstration and/or validation of second generation process improvements and applicable batch record revisions to accommodate such are outside the scope of this agreement. |
2.9.8. | Minimum Purchase Requirements (Exhibit B) shall be adjusted by mutual agreement based on the impact of the duration of the Stoppage. |
2.10. | Use and Purification of Starting Materials. |
2.10.1. | Cayman shall use all commercially reasonable efforts to efficiently purify starting materials with target yields provided in Exhibit C. Cayman shall |
6
provide reprocessing metrics (in a mutually agreed upon format) and batch documentation upon request.
2.10.2. | Cayman shall use all commercially reasonable efforts to make efficient use of starting materials supplied by Aerie (or by third-party suppliers on Aerie’s behalf, “Aerie Vendors”) and to maximize the amount of Product manufactured therefrom. Target quantities for starting materials to be consumed per kilogram of applicable Intermediate are provided in Exhibit C. |
2.10.3. | In the event that Cayman renders any portion of starting material, Intermediate, or Product un-recoverable at any step due to Gross Negligence, deliberate omission, or any willful failure to comply with the terms of this Agreement; Cayman shall be responsible for the cost of the portion of starting material(s) consumed through the applicable step. |
2.11. | Waste Streams. |
If requested, specific waste streams may be collected, further processed, transferred to the non-GMP process laboratory for evaporation, then shipped as-is to Aerie. Cayman shall use all commercially reasonable efforts to accommodate waste stream collections.
2.11.1. | Waste streams include: |
2.11.1.1. | Filtrates from the Step 1-2 [***]. |
2.11.1.2. | Collection of additional fractions following elution of the Step 3 [***]. |
2.11.1.3. | Filtrates from the Step 4 [***]. |
2.11.1.4. | Filtrates from the Step 5 [***]. |
2.11.2. | For Step 1-2 and Step 3 |
2.11.2.1. | Aerie shall submit a written request for specific waste streams with the Binding Forecast. |
2.11.2.2. | Aerie shall approve all applicable waste stream Exhibits prior to the targeted start of manufacturing. |
2.11.2.3. | Collection of the Step 3 waste stream may be limited by the availability of the applicable solvent. Cayman shall communicate any such vendor supply issue to Aerie, upon receiving notice from the vendor. |
2.11.3. | For Step 4 and Step 5 |
2.11.3.1. | Aerie shall submit a request for specific waste streams ten (10) business days prior to the start of the applicable step. |
2.11.3.2. | Aerie shall approve all applicable waste stream Exhibits prior to the start of manufacturing of applicable step. |
2.11.4. | All costs associated with collection, isolation, evaporation, analytical testing, storage, shipping of waste streams, and applicable batch record revisions to accommodate such are outside the scope of this agreement. |
2.12. | Inventory. |
7
Cayman shall provide monthly inventory reports, in a form mutually acceptable to Cayman and Aerie, which accounts for all Aerie inventory including: (i) incoming starting materials, (ii) purified and released starting materials, (iii) Intermediates, and (iv) Product. Inventory reports shall be provided within three (3) business days from the beginning of the month. Cayman will also allow Aerie, or its representatives, to conduct on site physical inventory count verifications at minimum on an annual basis, this may be concurrent with an Aerie audit of the Facility per Section 3.5 or following not less than thirty (30) days advance notice. Additional on-site physical inventory count verifications are also allowed by mutual agreement.
2.13. | Shipping Terms. All shipments shall be CIP “Destination” (Incoterms 2010) to an airport (in the case of shipment by airfreight) or to an address (in the case of shipment by courier service). The manner of shipment shall be designated by Cayman and the airport or address shall be designated by Aerie. Aerie shall provide the appropriate information regarding the broker (airport) or contact (final destination) prior to shipment, at minimum inclusive of a person’s name, e-mail address, and telephone number. All costs for such shipments shall be invoiced to Aerie following each shipment. |
Requests for shipping with notification less than ten (10) business days in advance of the requested shipping date shall be considered “Rush Shipping”. Rush Shipping is not guaranteed to be available, but Cayman shall use all commercially reasonable efforts to fulfill this request. Rush shipping may incur additional fees.
2.14. | Payment Terms. All payments from Aerie hereunder shall be made in U.S. dollars, by direct bank transfer, wire transfer, or check to an account designated in Cayman’s invoice. Payment terms shall be net thirty (30) days from the latter of date of invoice or date of order fulfillment. All undisputed amounts due under this Agreement shall, if overdue, bear interest until payment at a per annum rate which will be the sum of five percent (5%) and the prime rate in effect at the Bank of America or its successor on the due date, but in no event more than the maximum rate permitted by law. The payment of such interest shall not foreclose Cayman from exercising any other rights it may have resulting from any late payment. |
2.15. | Taxes. Aerie shall be responsible for the payment of any taxes, tariffs, or duties pursuant to CIP Destination (Incoterms 2010) related to the import of the Product into the country of destination. |
2.16. | Purchased Equipment. |
2.16.1. | The Parties acknowledge and agree that the equipment listed on Exhibit D (“Aerie Purchased Equipment”) was purchased by and is the property of Aerie, and that the Aerie Purchased Equipment has been installed at the Facility and suitably validated and/or qualified for use by Cayman. The Parties intend such Aerie Purchased Equipment shall remain at the Facility and be used by Cayman to manufacture Product during the Term. Cayman shall use the Aerie Purchased Equipment solely in connection with fulfilling its obligations pursuant to this Agreement and for no other purpose. |
2.16.2. | Cayman shall be responsible for reasonable upkeep and repair of the Aerie Purchased Equipment in line with regular maintenance and calibration schedules and abide by equipment supplier servicing timeframes. |
2.16.3. | In the event that the Aerie Purchased Equipment must be replaced, Cayman will be responsible for the cost of replacement of such Aerie Purchased Equipment and installation of new equipment as defined in Exhibit D. All replacements to the Aerie Purchased Equipment at Cayman’s expense will become Cayman’s property. |
8
2.16.3.1. | In the event a replacement is necessary the approval of such replacement shall not be unreasonably withheld by Cayman. |
2.16.3.2. | Following such approval, the timeline and, if applicable, delays from the manufacturer or supplier of the replacement equipment that impact Cayman’s ability to supply and Aerie’s ability to purchase shall not be considered a breach of contract under Section 7.3 and 7.5 of this agreement, unless equipment failures and associated stoppages are due to Cayman’s Gross Negligence or failure to comply with 2.16.2. |
2.16.3.3. | Cayman will not without Aerie’s prior written consent move the Aerie Purchased Equipment from the Facility and shall ensure that at all times it is only for use to supply Product to Aerie and its Affiliates. |
2.17. | Cost Adjustments. Should the production cost change considerably to the disadvantage of one of the Parties, the Party seeking a price adjustment shall provide documented evidence of the relevant factors (such as changes in the costs of raw materials, labor, utilities, or other overhead costs associated with the manufacture of the Product) and the Parties shall negotiate to solve such a problem in good faith, balancing the interests of the Parties, and shall mutually agree to any changes in writing. Any agreement between the Parties to change the price shall take effect from the beginning of the following Calendar Year (the “Subsequent Year”). If the Parties are unable to agree to change the price (or to leave it unchanged) before the start of the Subsequent Year, the price for Product shall be the same as that prevailing in the Calendar Year in which the price review discussions commenced. Notwithstanding the above, Cayman and Aerie shall cooperate to identify opportunities to reduce the cost of manufacturing and packaging Product. Cayman will implement process improvements at Aerie’s direction, following applicable risk assessment and determination of impact on regulatory filings, and share the benefits of price improvements with Aerie |
2.18. | Insurance. Cayman shall obtain and keep in force during the Term, at its sole cost and expense, such types and amounts of insurance as are customary for API manufacturers of Cayman’s size. At Aerie’s request, Cayman shall provide certificates of insurance documenting Cayman’s insurance coverage. |
2.19. | Business Review Meetings. Cayman and Aerie shall make reasonable best efforts to have in-person Business Review Meetings with a minimum frequency of not less than two (2) per year at a mutually agreed upon location. |
ARTICLE 3.0 QUALITY
3.1. | Quality. All Product supplied by Cayman shall meet (i) the requirements of the QTA, (ii) the current approved Specifications (as referenced in Exhibit A), (iii) additional requirements that the Parties may mutually agree to from time to time if technically feasible and in accordance with the change control procedure set out in the QTA, and (iv) the requirements of any Regulatory Authority to which Aerie has submitted, or notifies Cayman it will submit or sponsor the submission of, a Marketing Application. In case any official monograph or Regulatory Authority requirement conflicts with the current Specifications and Cayman’s manufacturing and control process of Product described in the MBR, the Parties will consult to seek a mutually acceptable solution. All Product supplied by Cayman shall be manufactured in accordance with CGMP, at Cayman’s Facility. Cayman shall maintain and comply with a quality management system and generate and maintain records of the manufacture of Product in accordance with the QTA. |
3.2. | Quality Control. |
9
3.2.1. | Starting Materials. Within forty-five (45) days of receipt by Cayman of a bulk or pre-shipment sample of a starting material provided by Aerie or an Aerie Vendor, Cayman shall perform quality control procedures to verify that all such starting materials conform fully to the specifications therefore and are otherwise suitable for use in the manufacture of Product. If Cayman determines that any starting materials fail to conform to their Specifications or are otherwise unsuitable for use in the manufacture of Product, Cayman shall notify Aerie and the vendor immediately (and, in any event, within two (2) business days of such determination), and shall provide Aerie with test results, representative samples, and any other evidence necessary to demonstrate such non-conformance or unsuitability, if requested. Cayman shall properly store and maintain all conforming starting materials to ensure they remain in compliance with Specifications and suitable for manufacture of Product. In the event a starting material from Aerie or an Aerie Vendor is rejected by Cayman, Aerie shall arrange for the return and replacement of such material and all such activities and related expenditures for such activities are at the expense of Aerie. |
3.2.2. | Product. Prior to each shipment of Product, Cayman shall perform quality control procedures to verify that the quantity or batch of such Product to be shipped conforms fully to the Specifications and additional requirements of Aerie as stipulated in Section 3.1. Each shipment of Product shall be accompanied by a COA describing all current requirements of the Specifications and results of tests performed. |
3.2.3. | Stability Testing. Cayman shall conduct stability testing of one (1) batch of the Product annually and shall provide Aerie with on-going stability reports. The cost of all such analysis and evaluations shall be borne by Cayman. |
3.3. | Rejection. Aerie shall have forty-five (45) days following its receipt of a shipment of Product (or, with respect to any Latent Defect, thirty (30) days following discovery by Aerie of such Latent Defect) to reject such Product on the grounds that all or part of the shipment fails to conform to the applicable Specifications or otherwise fails to conform to the warranties given by Cayman in Section 5.1, which rejection shall be accomplished by giving written notice to Cayman specifying the manner in which all or part of such shipment fails to meet the foregoing requirements. If rejection is based on grounds of contamination or Product not passing any physical test or Specification, rejection notice shall be accompanied by satisfactory representative data or sample provided to Cayman to verify such non-conformity (necessity of data or sample shall be mutually agreed between the Parties). If Aerie rejects a shipment before the date on which payment therefore is due, it may withhold payment for such shipment or the rejected portion thereof. The warranties given by Cayman in ARTICLE 5.0 below shall, to the extent provided therein, survive any failure to reject by Aerie under this Section 3.3. In the event of a rejection pursuant to this Section 3.3, at Aerie’s (or its relevant Affiliate’s) request, Cayman will deliver a replacement delivery of the Product to Aerie or its Affiliate as soon as practicable after notification of the rejection, using commercially reasonable efforts to ensure continuity of supply, and Aerie or the relevant Affiliate shall pay Cayman for such delivery in accordance with the payment provisions set out in this Agreement. |
3.4. | Returns and Settlement of Claims. Cayman shall be obliged to respond in writing to Aerie accepting or refusing a rejection notice from Aerie within thirty (30) days from the date of receipt of such rejection notice in accordance with Section 3.3, above. In case of disagreement between the Parties, the claim shall be submitted for tests and decision to an independent testing organization which meets appropriate GMP or consultant of recognized repute within the United States or EU pharmaceutical industry mutually agreed upon by the Parties (or, in the absence of such agreement, nominated by the President of the International Chamber of Commerce or his |
10
designee upon the application of either Party) (hereinafter referred to as the “Laboratory”), the appointment of which shall not be unreasonably withheld or delayed by either Party. The determination of such entity with respect to all or part of any shipment of Product shall be final and binding upon the Parties. The fees and expenses of the Laboratory making such determination shall be paid by the Party against which the determination is made (i.e., the Party whose argument is rejected by the Laboratory). Product accepted by Cayman as not meeting the applicable requirements and Specifications or so decided by the Laboratory shall be returned by Aerie to Cayman at Cayman’s expense. Cayman shall use its best efforts to replace the quantities of Product returned by Aerie within the shortest possible time, but no later than one hundred twenty (120) days from the return of such quantities unless mutually agreed upon by the Parties (e.g., if a Batch of Product is already underway in the Facility, such agreement shall not be unreasonably withheld). The replacement of returned Product shall be scheduled in such a way as to minimize the impact upon Cayman’s obligations for timely fulfillment and delivery of other Product ordered for shipment. Without limiting the remedies of Aerie, if Cayman fails to replace returned Product within one-hundred and fifty (150) days from the date Product is returned to Cayman, unless mutually agreed as stated above, Aerie shall have the right (i) to cancel such replacement shipment by written notice to Cayman, (ii) to reclaim immediately (either through refund or setoff, at Cayman’s option) the amounts paid pursuant to Section 2.6 above for the Product that was returned but not replaced, if such payment for such Product had already been made to Cayman accordingly, or (iii) or to have Cayman reprocess the material following mutual agreement.
3.5. | Aerie Audit of Facility. Upon advance notice given by Aerie to Cayman at a reasonable frequency, as described in the QTA, Aerie shall have the right to assign a reasonable number of employees or consultants of Aerie to inspect and audit the Facility at which Product is manufactured in order to verify Cayman’s compliance with the CGMP and other agreed or legal requirements, provided, however that (i) such employees or consultants shall not unreasonably interfere with activities being carried out of Facility, (ii) that such employees or consultants shall observe all rules and regulations generally applicable to visitors and to individuals employed at the Facility, and (iii) all such activities and related expenditures for such activities are at the expense of Aerie, Cayman shall have no monetary obligation resulting from such activities. Cayman employees shall cooperate fully with Aerie’s employees and consultants during any such inspections. For the avoidance of doubt, in conducting such inspections, Aerie employees and consultants may inspect, request samples, test, and analyze all manufacturing and packaging processes, equipment, and starting materials applicable to the Product and the Product itself. |
If such inspection or audit is performed as a “For-Cause” inspection (as described in the QTA) and reveals that Cayman is not in compliance with the CGMP or other agreed or legal requirements resulting in material quality or safety concerns, then Cayman shall reimburse Aerie for the cost of such audit and shall be responsible for all costs required to promptly remediate such critical non-compliance. In the event that Aerie identifies any such critical non-compliance in such audit and Cayman fails to implement any mutually agreed corrective or preventative action plan to remedy the same per the timeline in the same plan, and if the Parties are unable to agree a resolution such failure shall be considered a breach per Section 7.2 and subject to the applicable terms of the same except that the period of time that has run from the agreed completion date of any such corrective action plan shall be credited against the sixty (60) day notice provided for therein.
3.6. | Manufacturing Records. Cayman will maintain complete and accurate MBRs and any other manufacturing, processing, packaging, and quality control records (“Manufacturing Records”) sufficient to (i) show the complete history of Product manufacture, including batch numbers and production dates; (ii) facilitate easy identification and tracing of each lot, batch, unit production run, and any other applicable grouping; and (iii) include any other information reasonably requested by Aerie. Cayman shall make the Manufacturing Records and any other reports, evaluations, or other documents relating to the manufacture, storage, and packaging of Product |
11
available for inspection and copying at all times by Aerie or its authorized agents at the Facility and, at Aerie’s request, will deliver to Aerie a copy of all or any part of such records.
ARTICLE 4.0 REGULATORY MATTERS
4.1. | Inspections. Cayman shall permit Regulatory Authorities to conduct such inspections of the Facility as the Regulatory Authorities may request and shall cooperate with the regulatory agencies with respect to such inspections and any related matters. If any Regulatory Authority communicates to Cayman or Aerie (or its Affiliates) that they require any changes to be made with respect to the manufacture of the Product. The notified Party shall immediately notify the other Party and send to the other Party copies of any relevant documents delivered by said Regulatory Authority within five (5) business days from receipt. The Parties shall agree to an action plan with a target completion date and either (i) if as a result of any such change Aerie will need to alter any Marketing Authorization or commercial process used in the manufacture of finished products, defer the implementation of such change until Aerie can alter the affected Marketing Authorization and/or commercial process (unless and to the extent that such deferral is not reasonably practicable); or (ii) otherwise implement the relevant changes within the timeframe required by the Regulatory Authority and in accordance with the change control procedure in the QTA. |
4.2. | Aerie Cooperation. Aerie agrees to keep Cayman reasonably informed as to the status of the development of and applications for Marketing Authorizations filed in respect of the formulations incorporating the Product supplied hereunder to the extent such status may affect Cayman’s performance under this Agreement. |
4.3. | Cayman Cooperation. |
4.3.1. | Cayman shall promptly notify Aerie upon becoming aware of any problem related to the manufacture of Product such as where it may be affected by bacteriological or other contamination; significant chemical, physical, or other change; deterioration; stability failures; may not comply with the Specifications; or any other occurrence which might reasonably be expected to have adverse regulatory compliance and/or reporting consequences concerning the Product or Aerie’s formulations incorporating the Product. |
4.3.2. | Upon notification from Aerie or its Affiliates that it has received a complaint in respect of any of Aerie’s formulations incorporating the Product which may be related to the Product, Cayman shall promptly upon Aerie’s written request conduct all such necessary internal investigations as may be reasonably necessary to determine the validity of such complaint. The findings of such investigations shall be reported in writing to Aerie in accordance with the QTA. The costs of any such investigation which Aerie requires to be undertaken pursuant to this Section 4.3.2 shall be borne by Cayman in the event and to the extent that the need for such investigation is the result of a breach of Cayman’s warranties. |
4.4. | Maintenance of Approvals. Notwithstanding anything herein to the contrary, Cayman shall not undertake any modifications to the Facility, Product manufacturing or testing processes, Specifications, or filing that could impact Aerie Marketing Applications, Marketing Authorizations, regulatory product reviews, NDA, IND, or any other compliance status without prior agreement of Aerie. Notification concerning any significant change which could affect the aforementioned product approvals must be addressed solely to Aerie (as described in the Quality and Technical Agreement). |
4.5. | Regulatory Actions. Cayman will advise Aerie immediately, and in any event within two (2) business days, if Cayman receives any written notice from any Regulatory Authority or other third party that arises out of, directly relates to or affects Cayman’s performance under this |
12
Agreement, including: (i) any warning, citation, indictment, lawsuit, proceeding or claim that is issued, instituted or made by any Regulatory Authority; (ii) revocation of any license or permit or other document issued to Cayman; or (iii) any claim against Cayman for personal injury, death or property damage.
4.6. | Recalls. Cayman shall promptly notify Aerie upon becoming aware of any problem related to the manufacture of Product such as where it may be affected by bacteriological or other contamination, significant chemical, physical, or other change or deterioration or stability failures; may not comply with the Specifications, or any other occurrence which might reasonably be expected to have adverse regulatory compliance and/or reporting consequences concerning the Product’s or Aerie’s formulations incorporating the Product. |
Aerie will have sole discretion with regard to all decisions relating to whether to institute an inventory retrieval, recall, or any other action to stop the distribution and/or sale of the Product that does not meet the Specifications or pharmaceutical products comprising the Product (“Recalls”), as well as all decisions concerning any Recall strategy and execution. At Aerie’s request, Cayman will cooperate with Aerie in connection with any Recall, including coordinating with Aerie regarding any communication with local, state, or federal governmental agencies concerning a potential or actual Recall.
4.6.1. | If Aerie or any Regulatory Authority determines that a Recall is required, the Recall strategy shall be developed by Aerie and followed by Cayman, as applicable to the Product, with strict regard to timing requirements. The costs of any such Recall shall be borne by Cayman to the extent that the need for the action is the result of: |
4.6.1.1. | a manufacturing defect confirmed as a direct result of Cayman’s action(s) (including a Latent Defect) in the Product; |
4.6.1.2. | a failure on the part of Cayman to comply with its obligations under this Agreement; or |
4.6.1.3. | any Gross Negligence, deliberate omission, or Willful Misconduct on the part of Cayman. |
ARTICLE 5.0 PRODUCT WARRANTIES
5.1. | Process and Product Warranties. Cayman warrants and represents that: |
5.1.1. | Specifications. All Product supplied to Aerie hereunder shall comply with the Specifications for the Product, shall be free from contaminants, and shall conform with the information shown on the COA provided for the particular shipment according to Sections 3.1 and 3.2 hereof; |
5.1.2. | GMP. The Facility, and all Product supplied to Aerie hereunder meets and shall at all times meet (i) all US regulatory requirements for commercialization of the Product as an API, compliance with CGMP, demonstration of commercial production capability, and demonstration of acceptable stability of such Product; and (ii) all requirements imposed by other Regulatory Agencies in the Territories; |
5.1.3. | USP. All Product supplied to Aerie hereunder shall meet all compendia requirements as eventually promulgated by the USP and other applicable standards and shall be fit for human use; |
5.1.4. | Notification. Cayman will provide written notice according to Section 4.4 to Aerie of any proposed alterations to the Facility or to any Product manufacturing or testing process. |
13
5.1.5. | No Encumbrance. Title to all Product supplied to Aerie hereunder shall pass to Aerie as provided herein free and clear of any security interest, lien, or other encumbrance. |
5.1.6. | Regulatory. Cayman both warrants that they are not debarred under the U.S. Generic Drug Enforcement Act of 1992 and knowingly does not employ within the GMP division or use the services of any individual who is debarred or who has engaged in activities that could lead to being debarred. |
It has received no adverse communication from any Regulatory Authority in relation to the Facility and so far as it is aware, there are no grounds on which any Regulatory Authority could issue an adverse communication in relation to the Facility.
5.1.7. | IP. So far as it is aware, as of the date of this Agreement, the manufacture of the Product at the Facility does not infringe the Intellectual Property rights of any third party (provided that Cayman gives no representations or warranties in relation to the use by Cayman of any Aerie Intellectual Property in accordance with this Agreement). |
5.2. | Disclaimer. Neither Party shall be liable to the other hereunder for special, indirect, incidental, or consequential damages, whether in contract, warranty, negligence, tort, strict liability, or otherwise; provided that the foregoing limitation shall not apply to any such damages paid or payable to third parties in connection with an indemnifiable Claim pursuant to ARTICLE 10.0. |
5.2.1. | Exclusive Remedy. Except with respect to indemnification claims pursuant to Section 10.2, Aerie’s exclusive remedy and Cayman’s sole liability hereunder shall be limited to a refund of the purchase price and the cost of any starting materials provided by Aerie or Aerie Vendors and used by Cayman, or at Aerie’s option, the replacement, at no cost to Aerie (including, for the avoidance of doubt, reimbursement by Cayman to Aerie of the cost of any replacement starting materials from Aerie or Aerie Vendors required to manufacture replacement Product), of all material that does not meet the specifications. With respect to cases of rejection of Product by Aerie, said refund or replacement is conditioned on the requirements as stipulated in Section 3.3. |
ARTICLE 6.0 INTELLECTUAL PROPERTY
6.1. | The Parties acknowledge and agree that the IP subsisting at the Effective Date in the methods of manufacture of the Product, and any improvements to such methods made by Aerie or its Affiliates after the Effective Date, comprise Aerie’s IP. |
6.2. | Aerie hereby grants (and shall procure that each of its Affiliates grants) to Cayman a non-exclusive, royalty-free license to use for the sole purpose of manufacturing the Product for Aerie and otherwise performing its obligations and exercising its rights under this Agreement any of Aerie’s IP that would, but for the grant of such license, be infringed by Cayman in performing those obligations or exercising those rights or in so manufacturing the Product. Except as provided under this Section 6.2, nothing in this Agreement shall be construed as a transfer of or grant of rights by a Party or its Affiliates of any IP that they own, license, or control. |
6.3. | Cayman shall not challenge Aerie’s ownership of, or its right to use, Aerie’s IP. For the avoidance of doubt, any such challenge shall be a material breach of this Agreement. |
6.4. | If Cayman becomes aware at any time that any infringement or unauthorized use of any of Aerie’s IP is occurring, threatened, or likely, Cayman shall promptly provide to Aerie all such information as it has in relation thereto and all such assistance which Aerie reasonably requires in taking any action or proceedings in relation thereto. For the avoidance of doubt, Aerie shall have the exclusive right but not the obligation to take any action or proceedings against any third party in relation to Aerie’s IP. |
14
ARTICLE 7.0 TERM AND TERMINATION
7.1. | Term. This Agreement shall commence on the Effective Date and continue in full force until December 31, 2022 (the “Initial Term”) unless terminated earlier by mutual agreement of the Parties in writing or as otherwise provided in this Agreement. Following the Initial Term, Aerie may, at its option, renew the Agreement for up to two additional one (1) year periods by giving Cayman written notice of such election not less than sixty (60) days prior to the expiration of the Initial Term or the first renewal term, as applicable (each, a “Renewal Term” and, together with the Initial Term, the “Term”). |
7.2. | Breach. This Agreement may be terminated by either Party if the other Party breaches any material term or condition of this Agreement and fails to remedy the breach to the reasonable satisfaction of the non-breaching party within sixty (60) days after being given written notice thereof. |
7.3. | Failure to Supply. Without prejudice to Aerie’s right to terminate pursuant to Section 7.2, in the event that Cayman fails to fulfill a purchase order for Product that was properly submitted and accepted by Cayman pursuant to the terms of this Agreement within thirty (30) days of the delivery date specified in the purchase order or supplies Product that does not conform to specification, even if the purchase order for the Product was fulfilled on time: (i) Aerie shall have the right, at its option, to terminate this Agreement, and alternatively or in addition (ii) Aerie shall have no obligation to meet the Minimum Purchase Requirements for the duration of the next twelve (12) months from the agreed upon fulfillment date specified within the purchase order during which Cayman fails to fulfill such purchase order for Product. |
7.4. | Regulatory Issues. Without prejudice to its other rights and remedies, Aerie may terminate this Agreement by notice in writing to Cayman at any time if: (i) Aerie’s application for Marketing Authorization in the United States is rejected, (ii) any Regulatory Authority causes the clinical hold or permanent withdrawal of the Product, or (iii) if Cayman is debarred under the U.S. Generic Drug Enforcement Act of 1992, or (iv) if Cayman fails to gain regulatory approval for their Facility or such approval is revoked. |
If this agreement is terminated by Aerie for such a Regulatory Issue pursuant to Section 7.4 (i) or (ii), Aerie will pay to Cayman a Termination Fee per Section 7.8.1 save where such termination results from a Breach by Cayman of the terms of this Agreement.
7.5. | Failure to Purchase. In the event that Aerie fails to place purchase orders necessary to meet the Minimum Purchase Requirements (MPR) for a given Year (Section 2.2.1 and Exhibit B) following any necessary adjustment per Section 2.9.8, save where such failure results from a breach by Cayman of any term of this Agreement, Cayman shall invoice Aerie a Failure to Purchase Fee (FTPF) for the material difference between the actual purchased quantity (APQ) and the MPR for the applicable year equal to the greater of the amounts calculated using the following two formulas: |
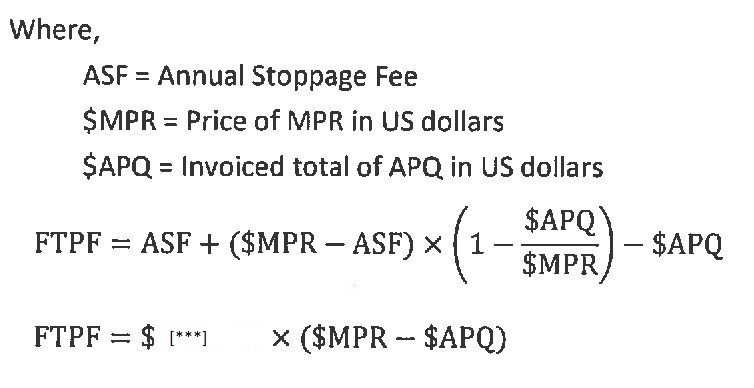
15
7.5.1. | Aerie shall pay such invoice as provided in Section 2.14. This Section 7.5 shall not apply in the event of termination of this Agreement, where Cayman’s sole remedy shall be any Termination Fee payable pursuant to Section 7.8.1. |
7.6. | Either Party may request an adjustment to the Minimum Purchase Requirements in Exhibit B by giving notice in writing (a “Review Notice”) to the other Party at least three (3) months before the expiry of the two (2) year period commencing on the Effective Date (the “Initial Period”); or the expiry of each subsequent two (2) year period after the Initial Period (each a “Review Date”). A Review Notice may request an adjustment to the Minimum Purchase Requirements in order to reflect changes in end user demand for the drug product. Following receipt of a Review Notice, representatives of the Parties shall meet to review and discuss the same and shall negotiate in good faith to agree revised Minimum Purchase Requirements. Any revised Minimum Purchase Requirements agreed pursuant to this Section shall take effect from the relevant Review Date, unless otherwise agreed. In the event the Parties are unable to reach agreement in relation to a Minimum Purchase Requirements adjustment requested by either Party in accordance with this Section, the Parties shall use commercially reasonable efforts to resolve the dispute by prompt discussion in good faith at a managerial level appropriate to the dispute. If the Dispute has not been resolved within ten (10) business days, the Minimum Purchase Requirements shall remain unchanged and either Party may terminate the Agreement by giving at least six (6) months’ prior written notice of such termination to the other Party. |
In the event that the new Minimum Purchase Requirements or such notice of termination results in a Stoppage, fees per Section 2.9.5 shall apply.
7.7. | Effect of Termination. In the case of termination by a Party under this ARTICLE 7.0 at such Party’s option, the Parties’ obligations, including Cayman’s obligation to supply Product ordered by Aerie prior to the effective date of such termination, and Aerie’s obligation to purchase Product included in any binding forecast pursuant to Section 2.3.1 shall survive termination. In addition, Aerie may purchase, and Cayman agrees to supply quantities of Product for which Aerie has not found alternate suppliers for one year following such termination, at then current prices of Product and pursuant to purchase orders issued outside of this Agreement. |
Following completion of Cayman’s obligations, Aerie shall have ninety (90) days to arrange for removal of the Aerie Purchased Equipment (Section 2.16); such removal and related expenditures for such activities are at the expense of Aerie, Cayman shall have no monetary obligation resulting from such activities. Cayman shall allow Aerie to access the Facility to remove such Aerie Purchased Equipment and provide reasonable assistance in relation to such removal. Any Aerie Purchased Equipment which remains in the Facility after ninety (90) days shall become the sole property of Cayman. Notwithstanding the foregoing, if this Agreement is terminated by reason of the breach by Aerie of its obligations with respect to the Minimum Purchase Requirements, Cayman may retain the Aerie Purchased Equipment as additional remedy for any such breach.
7.8. | Termination Fee. Save where such termination results from a breach by Cayman of the terms of this Agreement (in which case no fee shall be payable), Aerie shall pay to Cayman a Termination Fee. |
7.8.1. | In the case of termination by Aerie prior to the expiry of the Initial Term pursuant to Section 7.4(i) or 7.4(ii), the Termination Fee shall be calculated [***]. |
7.8.2. | In the case of termination by Aerie prior to the expiry of the Initial Term pursuant to Section 7.6, the Termination Fee shall be calculated [***]. |
7.8.3. | Cayman shall use commercially reasonable efforts to re-allocate capacity to third parties and otherwise mitigate its losses. |
16
7.8.4. | In the event Cayman is able to re-allocate capacity to third parties per Section 7.8.3, Cayman shall refund to Aerie the applicable portion of the Termination Fee already paid for the number of months of the initial term that the dedicated GMP Suite will be re-allocated. |
7.9. | Survival. It is understood that termination or expiration of this Agreement shall not relieve a Party from any liability which, at the time of such termination or expiration, has already accrued to the other Party. The provisions of ARTICLE 1.0, ARTICLE 4.0, ARTICLE 5.0, ARTICLE 7.0, ARTICLE 8.0, ARTICLE 9.0, ARTICLE 10.0, and ARTICLE 11.0 shall survive the termination of this Agreement for any reason. Except as otherwise expressly provided in this Agreement, all other rights and obligations of the Parties shall cease upon termination of this Agreement. |
ARTICLE 8.0 CONFIDENTIALITY AND EXCLUSIVITY
8.1. | Confidential Information. |
8.1.1. | The Parties entered into a Confidentiality Agreement dated on April 1, 2011. The Parties acknowledge that the Confidential Information shared by them that is the subject matter of this Manufacture and Supply Agreement, as well as the original Confidentiality Agreement, continue to be a valuable and unique asset to both. For ease of reference, the Parties wish to reiterate the principles surrounding the manner by which Confidential Information will be shared by both Parties here and desire to continue to abide by these principles throughout this Manufacture and Supply Agreement. |
8.1.2. | “Confidential Information” shall mean information pertaining to Cayman’s and/or Aerie’s (i) intellectual property, including without limitation, patents, patent applications, copyrights, copyright applications and trade secrets and/or (ii) confidential information, including without limitation, ideas. Drug Master Files (DMF), synthetic procedures, analytical testing procedures and results, processes, and methods of production/manufacturing, new chemical entities, reagents, chemical compounds, assays, techniques, sketches, schematics, drawings, works of authorship, models, designs, inventions, know-how, technical documentation, processes, apparatuses, equipment, algorithms, software programs, software source documents, formulae, information concerning research projects, experimental work, development, design details and specifications, engineering and works in process, future developments relating to the current, future and proposed products and services, financial information, information relating to procurement requirements, purchasing, manufacturing, customer lists, product plans, product ideas, business strategies, marketing or business plans, financial or personnel matters, investors, employees, business and contractual relationships, business forecasts, sales and merchandising, and information regarding affiliates, third parties, suppliers, customers, employees or investors, whether in written, oral, graphic, or electronic form, synthetic procedures, processes, products methods of production/manufacturing and information regarding customers and materials. Confidential Information may be furnished to each other in written, graphic, or oral form, either directly or indirectly. |
8.1.3. | Confidential Information in this Agreement does not comprise the following: |
8.1.3.1. | Information that is now proven to be in the public domain or proven to subsequently have been entered in the public domain through legal means, and also without fault on the part of either Party. |
17
8.1.3.2. | Information that demonstrably was known to Cayman or Aerie prior to receipt from each other; or |
8.1.3.3. | Information that can be proven by Cayman or Aerie that has been received from any third party having a lawful right to disclose such information. |
8.1.3.4. | Information that can be proven by Cayman or Aerie that has been independently discovered or developed without the use of the other Party’s information. |
For the purpose of the provisions of this paragraph, disclosures made to Cayman and/or Aerie under this Agreement which are specific, for example, as to products, techniques, etc., shall not be deemed to be within the foregoing exceptions merely because they are embraced by general disclosures in the public domain or in the possession of Cayman or Aerie. In addition, any combination of features shall not be deemed to be within the foregoing exceptions merely because individual features are in the public domain or in the possession of Cayman or Aerie.
8.1.4. | Cayman and Aerie agree to maintain secrecy of all Confidential Information. In this regard, Cayman and Aerie agree to disclose all necessary Confidential Information and to provide to only to those Cayman or Aerie officers and employees who are directly concerned with the use and evaluation of said Confidential Information for the purpose specified above and who are bound by the same secrecy obligations hereunder. Cayman and Aerie shall take all necessary and reasonable precautions to prevent such Confidential Information from being disclosed or provided to any unauthorized person, firm, or company. |
8.1.5. | The receiving Party shall, if so requested by any court of competent jurisdiction or any competent judicial, governmental, or regulatory body, be entitled to disclose to such body the Confidential Information requested provided that the receiving Party shall prior to such disclosure use its reasonable endeavors to inform the other Party of the full circumstances and the Confidential Information that will be disclosed and shall further only disclose that part of the Confidential Information requiring disclosure. If the receiving Party is unable to information the other before the Confidential Information is disclosed pursuant to this paragraph it shall to the extent permitted by law inform the other of the full circumstances of disclosure and the Confidential Information which has been disclosed immediately after the disclosure. |
8.1.6. | Cayman and Aerie agree not to use the other Party’s Confidential Information for any reason other than for the Purpose stated above without first obtaining the other Party’s express written consent. |
8.1.7. | If the recipient of the Confidential Information breaches any of the above provisions of this Agreement, the receiving Party agrees that the disclosing Party will suffer immediate and substantial damage and that monetary damages will not be an adequate remedy. Upon the occurrence of any such breach. Such injunctive relief, however, shall not preclude the disclosing party form seeking monetary damages arising out of such breach. In the event of a material breach by the receiving Party of this Agreement, the receiving Party agrees to pay any fees and expenses incurred by the disclosing Party enforcing this Agreement. |
8.2. | No Use of Name. Without the other Party’s prior written consent, neither Party shall use the name, trademarks, or trade dress in any sales or advertising material, or otherwise disclose to any third party the fact that Cayman manufactures and supplies Product to Aerie or the identity or properties of such Product. |
18
8.3. | Equitable Relief. The Parties acknowledge that the unauthorized use or disclosure of the other party’s Confidential Information may cause that Party irreparable harm and that money damages may be inadequate to compensate that Party for such harm. Accordingly, in addition to any other available remedies, that Party shall be entitled to seek equitable relief, including injunctive relief and/or specific performance, the granting of which shall not be subject to or conditioned upon any requirement of posting a bond or other security. |
8.4. | Exclusivity. In recognition of the substantial investment being made by Aerie in establishing the arrangements with Cayman contemplated by this Agreement, the development of substantial manufacturing know-how for the Product at Aerie’s expense, Aerie’s purchase of certain capital equipment, and the provision by Aerie of technical assistance, know-how and Confidential Information to facilitate the manufacture and supply of Product, Cayman agrees that it will not directly, or indirectly through a third party, manufacture, supply, or sell Product, Intermediates thereof, or any other product using or in any way derived from Aerie’s Confidential Information, in any form to any person or entity other than Aerie or its designees for so long as this Agreement remains in effect. |
ARTICLE 9.0 REPRESENTATIONS AND WARRANTIES
9.1. | Cayman. Cayman represents and warrants that: (i) it has full power to enter into this Agreement; (ii) it has obtained all necessary corporate approvals to enter into and execute the Agreement; (iii) it has not entered and will not enter into any agreements with any third Party that are inconsistent with this Agreement; (iv) Cayman shall fully comply with the requirements of any and all applicable federal, state, local, and foreign laws, regulations, rules, and orders of any governmental body having jurisdiction over the activities contemplated by this Agreement; and (v) that the provisions of this Agreement, and the rights and obligations of the Parties hereunder, are enforceable against Cayman, subject to laws respecting creditors’ rights and general principles of equity. |
9.2. | Aerie. Aerie represents and warrants that: (i) it has full power to enter into the Agreement; (ii) it has obtained all necessary corporate approvals to enter into and execute this Agreement; (iii) it has not entered and will not enter into any agreements of any third Party that are inconsistent with this Agreement; (iv) so far as it is aware as at the date of this Agreement the manufacture of the Product at the Facility does not infringe the Intellectual Property rights of any third party; and (v) Aerie shall fully comply with the requirements of any and all applicable local and foreign laws, regulations, rules, and orders of any governmental body having jurisdiction over the activities contemplated by this Agreement; and (vi) that the provisions of this Agreement, and the rights and obligations of the Parties hereunder, are enforceable against Aerie, subject to laws respecting creditors’ rights and general principles of equity. |
9.3. | Disclaimer. EXCEPT AS PROVIDED IN THIS ARTICLE 9.0 AND ARTICLE 5.0 ABOVE, NEITHER PARTY MAKES ANY WARRANTIES OR CONDITIONS (EXPRESS, IMPLIED, STATUTORY, OR OTHERWISE) WITH RESPECT TO THE SUBJECT MATTER HEREOF. |
ARTICLE 10.0 INDEMNIFICATION
10.1. | Aerie. Aerie shall indemnify, defend and hold harmless Cayman, their directors, officers, employees, agents, successors, and assigns from and against any liabilities, expenses, or costs (including reasonable attorneys’ fees) arising out of any claim, complaint, suit, proceeding, or cause of action (collectively, “Claims”) against any of them by a third party (whether alleging physical injury or death or otherwise) resulting from (i) the clinical testing of the finished dosage incorporating the Product by or on behalf of Aerie; (ii) manufacture and handling of the Product on behalf of Aerie and its Affiliates when in accordance with the safety data provided by Aerie to Cayman (iii) the safety of the finished dosage incorporating the Product distributed by or on |
19
behalf Aerie; (iv) the promotion, distribution, sale, handling, possession, or use of the finished dosage incorporating the Product by or on behalf of Aerie following its or their acceptance thereof in accordance with Section 3.3 above; (v) an act or omission of Aerie that constitutes Gross Negligence or is intentionally wrongful; and (vi) any material breach by Aerie of its representations and warranties under Section 9.2 above, in each case subject to the requirements set forth in Section 10.3 below and except, in each case, to the extent that Cayman is obligated to indemnify Aerie for such Claim pursuant to Section 10.2.
10.2. | Cayman. Cayman shall indemnify, defend and hold harmless Aerie and its Affiliates, their directors, officers, employees, agents, successors, and assigns from and against all liabilities, expenses, and costs (including reasonable attorneys’ fees) arising out of any Claim, against any of them by a third party (whether alleging physical injury or death or otherwise) resulting from (i) an act or omission of Cayman that constitutes Gross Negligence or is intentionally wrongful; (ii) any loss of Product for which Cayman bears the risk under Section 2.7; and (iii) any breach by Cayman of any of its representations and warranties under Section 5.1 or 9.1, in each case subject to the requirements set forth in Section 10.3 below and except, in each case, to the extent that Aerie is obligated to indemnify Cayman for such Claim pursuant to Section 10.1). |
10.3. | Indemnification Procedure. Any Party seeking indemnification under this ARTICLE 10.0 (the “Indemnitee”) shall promptly notify the indemnifying Party (the “Indemnitor”) of such Claim, provided that any failure to so notify shall not affect a Party’s right to indemnification except to the extent that such failure materially prejudices the ability of the Indemnitor to defend against such Claim. At the Indemnitee’s option, the Indemnitee may (i) retain sole control over defense and settlement of the Claim, provided that Indemnitee shall not settle such Claim without the Indemnitor’s prior consent, not to be unreasonably withheld; or (ii) provide the Indemnitor sole control over the defense and settlement thereof, provided that Indemnitor shall not settle such Claim without the Indemnitee’s prior consent to the extent that such settlement requires any admission of liability or wrongdoing or the payment of any amount by Indemnitee. Without limiting the foregoing, with respect to Claims brought under Section 10.1 or 10.2 above and tendered to the Indemnitor pursuant to sub-section (ii) of the previous sentence: (i) at Indemnitor’s request and expense, Indemnitee shall provide full information and reasonable assistance to Indemnitor with respect to such Claims; and (ii) Indemnitee, at its own expense, shall have the right to participate with counsel of its own choosing in the defense and/or settlement of any such Claim. |
ARTICLE 11.0 GENERAL
11.1. | Assignment. The Parties agree that their rights and obligations under this Agreement may not be assigned or otherwise transferred to a third party without the prior written consent of the other Parties hereto. Notwithstanding the foregoing, either Party may transfer or assign any or all of its rights and obligations under this Agreement: (i) to an Affiliate; or (ii) to a successor to all or substantially all of its business or assets relating to this Agreement whether by sale, merger, operation of law, or otherwise; provided that such assignee or transferee has agreed in writing addressed to the other Party to be bound by the terms and conditions of this Agreement; such agreement from the Affiliate or successor shall not be unreasonably withheld. Subject to the foregoing, this Agreement shall be binding upon and inure to the benefit of the Parties hereto, their successors, and assigns. Any purported assignment in violation of this Section 11.1 shall be null and void. |
11.2. | Governing Law. This Agreement shall be governed by and interpreted in accordance with the laws of State of Delaware, U.S.A. (without giving effect to any conflicts of laws rules thereof or of any other jurisdiction). Any dispute in relation to this Agreement shall be subject to the exclusive jurisdiction of the federal and state courts of the State of Delaware, U.S.A. |
20
11.3. | Notices. Any notice or report required or permitted to be given or made under this Agreement by either Party shall be in writing and delivered to the other Party at its address indicated below (or to such other address as a Party may specify by notice hereunder) by nationally recognized courier (e.g., FedEx) or by registered or certified mail, postage prepaid. All notices shall be effective as of the date delivered to the addressee. |
If to Aerie: | ||
Aerie Distribution, Inc. 0000 Xxxxxxx Xxxx. Xxxxx 000X Xxxxxx, XX 00000, XXX Attn: VP, Manufacturing | ||
CC: | ||
Aerie Pharmaceuticals 000 XX Xxxxxxx 000 Xxxxx 00 Xxxxxxxxxx, XX 00000 Xxxx: General Counsel | ||
If to Cayman: | ||
Cayman Chemical Company, Inc. | ||
0000 X. Xxxxxxxxx Xxxx | ||
Xxx Xxxxx, XX 00000, XXX | ||
Attn: Xxxxxxx Xxxxxx | ||
CC: Xxxxxxx Xxxxx | ||
11.4. | Limitation of Liability, SUBJECT TO THE LIMITATIONS OF SECTION 5.2, NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY OR ANY AFFILIATE FOR ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL, OR EXEMPLARY DAMAGES (INCLUDING, TO THE EXTENT INDIRECT, LOST OR ANTICIPATED REVENUES OR PROFITS RELATING TO THE SAME), ARISING FROM ANY CLAIM RELATING TO THIS AGREEMENT, WHETHER SUCH CLAIM IS BASED ON CONTRACT, TORT (INCLUDING NEGLIGENCE), OR OTHERWISE, EVEN IF AN AUTHORIZED REPRESENTATIVE OF SUCH PARTY IS ADVISED OF THE POSSIBILITY OR LIKELIHOOD OF SAME. THESE LIMITATIONS SHALL APPLY NOTWITHSTANDING THE FAILURE OF THE ESSENTIAL PURPOSE OF ANY LIMITED REMEDY, AND THE PARTIES ACKNOWLEDGE THAT THIS PARAGRAPH REPRESENTS A REASONABLE ALLOCATION OF RISK. |
11.5. | Force Majeure. None of the Parties will be liable for its failure to perform any of its obligations hereunder during any period in which such performance is delayed by acts of God, fire, war, embargo, riots, strikes, or other similar cause outside the control of such Party (a “Force Majeure Event”). In the event of either Party being so delayed or prevented from performing its obligations, such Party must: |
11.5.1. | give notice in writing of such delay or prevention to the other party as soon as reasonably possible stating the commencement date and extent of such delay or prevention, the cause of such delay or prevention, and its estimated duration; |
21
11.5.2. | use all reasonable endeavors to mitigate the effects of such delay or prevention upon the performance of its obligations under this Agreement; and |
11.5.3. | resume performance of its obligations as soon as reasonably possible after the removal of the cause of the delay or prevention. |
11.6. | If either Party is prevented from performing its obligations by a Force Majeure Event for more than sixteen (16) consecutive weeks (the “Affected Party”), the Affected Party shall be considered in breach of this agreement and subject to the terms of Section 7.2. |
11.7. | Headings. Headings included herein are for convenience only, do not form a part of this Agreement and shall not be used in any way to construe or interpret this Agreement. |
11.8. | Non-Waiver. Any waiver of the terms and conditions hereof must be explicitly in writing. The waiver by any of the Parties of any breach of any provision hereof by the others shall not be construed to be a waiver of any succeeding breach of such provision or a waiver of the provision itself. |
11.9. | Severability. Should any section or portion thereof of this Agreement (including the provisions of Section 8.4) be held invalid by reason of any law, statute, or regulation existing now or in the future in any jurisdiction by any court of competent authority or by a legally enforceable directive of any governmental body, such section or portion thereof shall be validly reformed so as to approximate the intent of the Parties as nearly as possible and render it enforceable to the maximum extent permitted by law and, if unreformable, shall be deemed divisible and deleted with respect to such jurisdiction, but the Agreement shall not otherwise be affected. |
11.10. | Independent Contractors. The relationship of Aerie and Cayman established by this Agreement is that of independent contractors. Nothing in this Agreement shall be construed to create any other relationship between Aerie and Cayman. Neither Party shall have any right, power, or authority to assume, create or incur any expense, liability, or obligation, express or implied, on behalf of the other. |
11.11. | Entire Agreement. The terms and provisions contained in the Agreement, including the Exhibits hereto, constitute the entire agreement between the Parties and shall supersede all previous communications, representations, agreements, or understandings, either oral or written, between the Parties with respect to the subject matter thereof. Notwithstanding the foregoing, no Party waives any rights it may have under the Supply Agreement. No agreement or understanding varying or extending this Agreement shall be binding upon any Party hereto, unless set forth in a writing which specifically refers to the Agreement signed by duly authorized officers or representatives of the respective Parties, and the provisions hereof not specifically amended thereby shall remain in full force and effect. |
11.12. | Counterparts. This Agreement may be executed in counterparts and by facsimile or other means of electronically imaging a signature, each of which shall be deemed an original, but which together shall constitute one and the same instrument. |
22
IN WITNESS WHEREOF, the Parties hereto have caused their duly authorized representatives to execute this Agreement.
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxxxx Xxxxxx | By: | /s/ Xxx Xxxxx | |
Xxxxxxx Xxxxxx | Xxx Xxxxx | |||
VP Quality and Regulatory Affairs | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:10 EDT | |
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxx X. Xxxxxxx | By: | /s/ Xxxx Xxxxxx | |
Xxxxx X. Xxxxxxx | Xxxx Xxxxxx | |||
Chief Financial Officer | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:17 EDT | |
23
EXHIBIT A
Cayman Specifications
Aerie API | Specification |
AR-13324 | Material Specification Sheet AD126 MAT |
This Exhibit A is agreed to by the Parties with an effective date of 01 January 2018.
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxxxx Xxxxxx | By: | /s/ Xxx Xxxxx | |
Xxxxxxx Xxxxxx | Xxx Xxxxx | |||
VP Quality and Regulatory Affairs | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:10 EDT | |
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxx X. Xxxxxxx | By: | /s/ Xxxx Xxxxxx | |
Xxxxx X. Xxxxxxx | Xxxx Xxxxxx | |||
Chief Financial Officer | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:17 EDT | |
24
EXHIBIT B
Minimum Purchase Requirements
Year | Minimum Quantity | Price per Kilogram1,2 |
2018 | [***] kg | Up to Minimum Quantity for applicable Year: $[***]/kg In excess of Minimum Quantity for applicable Year: $[***]/kg |
2019 | [***] kg | |
2020 | [***] kg | |
2021 | [***] kg | |
2022 | [***] kg | |
1 Invoiced amounts will be reflective of the quantity purchased to the nearest hundredth of a kilogram.
2 Aerie will be credited $[***] per each Aerie purchased [***].
3 If [***] Starting materials are used, Aerie will be credited $[***] per kg of API.
For purposes of clarity the per kilogram price for the minimum quantity ordered in any single year shall be $[***]/kg. After reaching this threshold, the per kilogram price shall be $[***]/kg.
Payment Schedule
Milestone | Milestone Payment |
Receipt of PO | Fifty Percent (50%) of the Price based on the ordered Quantity on the PO |
QA Release of Product | Balance of the Price due based on the Total Yield of the applicable Batch |
This Exhibit may be reviewed annually; the first such review period shall be the third quarter of 2019, and the third quarter of subsequent years. Review may be requested by either Party in the event of a significant change in process yield. If such review results in changes to the Price per Kilogram a corresponding change to the Minimum Quantity is merited.
[Signatures on the following page.]
25
This Exhibit B is agreed to by the Parties with an effective date of 01 January 2018.
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxxxx Xxxxxx | By: | /s/ Xxx Xxxxx | |
Xxxxxxx Xxxxxx | Xxx Xxxxx | |||
VP Quality and Regulatory Affairs | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:10 EDT | |
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxx X. Xxxxxxx | By: | /s/ Xxxx Xxxxxx | |
Xxxxx X. Xxxxxxx | Xxxx Xxxxxx | |||
Chief Financial Officer | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:17 EDT | |
26
EXHIBIT C
Starting Materials Usage:
Starting Material | [***]1 |
[***] | [***] |
1Usage is based on the material that passes the SM specification following any applicable reprocessing.
Starting Material Purification Yield:
Starting Material | Minimum Yield Targets1 for Purification of Starting Materials |
[***] | [***]% |
[***] | [***]% |
1Minimum Yield Target quantities are based on the assumption that the material received from the Aerie vendor is typical of material received and within specifications.
This Exhibit C is agreed to by the Parties with an effective date of 01 January 2018.
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxxxx Xxxxxx | By: | /s/ Xxx Xxxxx | |
Xxxxxxx Xxxxxx | Xxx Xxxxx | |||
VP Quality and Regulatory Affairs | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:10 EDT | |
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxx X. Xxxxxxx | By: | /s/ Xxxx Xxxxxx | |
Xxxxx X. Xxxxxxx | Xxxx Xxxxxx | |||
Chief Financial Officer | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:17 EDT | |
27
EXHIBIT D
Aerie Equipment
•[***] Reactor [***] (CAY15038)
•[***] (CAY15028)
•[***] (CAY15031)
•[***] Xxxxxx Filter and Spray Ball [***] (CAY15039)
•[***] Purified Water System (CAY15032)
•[***]
•Vacuum Oven (CAY15021)
•[***] Vacuum Pump (CAY15049)
•[***] Vacuum Pump (CAY15037)
•[***] Pneumatic Diaphragm Pumps (2)
•[***] Diaphragm Pump [***]
•[***] Desiccant Cabinet (CAY14033)
•[***] Data Acquisition and probes (CAY15083)
•[***] Precision Balance (CAY15022)
•[***] Precision Balance (CAY15023)
•[***] Precision Balance (CAY15026)
•[***] Wheeled Scale (CAY15025)
•[***] Stainless Steel Glove Box (CAY15046)
•[***] Temperature and Humidity Probes, Quantity 5
•[***] Gas Scrubber (CAY15050)
•[***] Freezer (CAY17034)
•[***] Filter Housing (CAY16036)
•[***] Mechanical Platform
•[***] (TBD)
•[***] (TBD)
[Signatures on the following page.]
28
Manufacture and Supply Agreement
This Exhibit D is agreed to by the Parties with an effective date of 01 January 2018.
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxxxx Xxxxxx | By: | /s/ Xxx Xxxxx | |
Xxxxxxx Xxxxxx | Xxx Xxxxx | |||
VP Quality and Regulatory Affairs | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:10 EDT | |
Cayman Chemical Company, Inc. | Aerie Distribution, Inc. | |||
By: | /s/ Xxxxx X. Xxxxxxx | By: | /s/ Xxxx Xxxxxx | |
Xxxxx X. Xxxxxxx | Xxxx Xxxxxx | |||
Chief Financial Officer | ||||
Date: | 20 Apr 2018 | Date: | 20-Apr-2018 18:17 EDT | |
29

